Pigment-dispersing factor neuropeptides act as multifunctional hormones and modulators in tardigrades
- PMID: 40037531
- PMCID: PMC11879619
- DOI: 10.1098/rsob.240242
Pigment-dispersing factor neuropeptides act as multifunctional hormones and modulators in tardigrades
Abstract
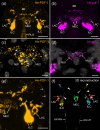
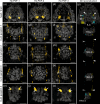
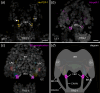
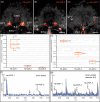
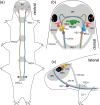
Pigment-dispersing factors (PDFs) are neuropeptides that play key roles in controlling the circadian rhythms in various insects, whereas their function remains elusive in other protostomes including tardigrades (water bears). Here we show that the three PDFs of the tardigrade Hypsibius exemplaris are co-localized in two pairs of inner lobe cells in the brain, whereas only one PDF occurs in four additional cerebral and two extracerebral cells. The axons of the inner lobe cells pass through the contralateral brain hemisphere, descend to the ventral nerve cord and terminate in two pairs of potential release sites in the posteriormost trunk ganglion. Using in vitro assays, we demonstrate that all three PDFs and their deorphanized receptor (PDFR) are functional. Widespread localization of PDFR suggests that tardigrade PDFs may act as multifunctional hormones and neuromodulators that control major functions including light detection, neural processing, locomotion, feeding, digestion, osmoregulation, growth, embryonic development and oogenesis/reproduction.
Keywords: G protein-coupled receptor; Hypsibius exemplaris; Tardigrada; gene expression; nervous system; neuromodulator.
Conflict of interest statement
We declare we have no competing interests.
Figures



References
-
- Homberg U, Würden S, Dircksen H, Rao KR. 1991. Comparative anatomy of pigment-dispersing hormone-immunoreactive neurons in the brain of orthopteroid insects. Cell Tissue Res. 266, 343–357. (10.1007/BF00318190) - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
