Applications of artificial intelligence and advanced imaging in pediatric diffuse midline glioma
- PMID: 40037540
- PMCID: PMC12309720
- DOI: 10.1093/neuonc/noaf058
Applications of artificial intelligence and advanced imaging in pediatric diffuse midline glioma
Abstract
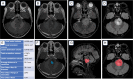
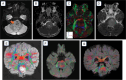
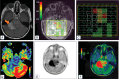
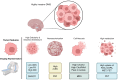
Diffuse midline glioma (DMG) is a rare, aggressive, and fatal tumor that largely occurs in the pediatric population. To improve outcomes, it is important to characterize DMGs, which can be performed via magnetic resonance imaging (MRI) assessment. Recently, artificial intelligence (AI) and advanced imaging have demonstrated their potential to improve the evaluation of various brain tumors, gleaning more information from imaging data than is possible without these methods. This narrative review compiles the existing literature on the intersection of MRI-based AI use and DMG tumors. The applications of AI in DMG revolve around classification and diagnosis, segmentation, radiogenomics, and prognosis/survival prediction. Currently published articles have utilized a wide spectrum of AI algorithms, from traditional machine learning and radiomics to neural networks. Challenges include the lack of cohorts of DMG patients with publicly available, multi-institutional, multimodal imaging and genomics datasets as well as the overall rarity of the disease. As an adjunct to AI, advanced MRI techniques, including diffusion-weighted imaging, perfusion-weighted imaging, and Magnetic Resonance Spectroscopy (MRS), as well as positron emission tomography (PET), provide additional insights into DMGs. Establishing AI models in conjunction with advanced imaging modalities has the potential to push clinical practice toward precision medicine.
Keywords: artificial intelligence; deep learning; diffuse intrinsic pontine glioma; diffuse midline glioma; radiomics.
© The Author(s) 2025. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
A.R.: Co-founder and consultant for MRIMatch, Inc., and consultant for Arterys, Inc.
Other authors declare no conflicts of interest.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

