In Silico Study of a Bacteriorhodopsin/TiO2 Hybrid System at the Molecular Level
- PMID: 40037620
- PMCID: PMC11948329
- DOI: 10.1021/acs.jctc.4c01370
In Silico Study of a Bacteriorhodopsin/TiO2 Hybrid System at the Molecular Level
Abstract
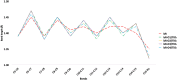
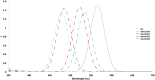
Bacteriorhodopsin (bR) is a light-harvesting membrane protein that represents a promising sensitizer of TiO2 for photovoltaic and photoelectrochemical devices. However, despite numerous experimental studies, the molecular-level understanding of the bR/TiO2 hybrid system is still unsatisfactory. In this contribution, we report the construction and analysis of an atomistic model of such a system. To do so, both steered molecular dynamics-molecular dynamics and quantum mechanics/molecular mechanics computations are applied to four different bR orientations on the anatase TiO2 surface. The resulting bR/TiO2 models are then used to compute the light absorption maxima changes relative to those of bR. We show that all four models reproduce the experimentally observed blue-shift value induced by bR binding on TiO2 and could be used to study the binding and binding-induced protein modifications. We conclude that the constructed models could provide a basis for future studies aiming to simulate the complex long-range electron transfer mechanism in bR/TiO2-based solar energy conversion devices as well as in engineering bR to achieve enhanced efficiencies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
