A novel human milk fortifier supports adequate growth in very low birth weight infants: a non-inferiority randomised controlled trial
- PMID: 40037774
- PMCID: PMC12418532
- DOI: 10.1136/archdischild-2024-327282
A novel human milk fortifier supports adequate growth in very low birth weight infants: a non-inferiority randomised controlled trial
Abstract
Objective: To compare growth, tolerance and safety parameters in very preterm infants receiving human milk (HM) fortified with a multicomponent cow's milk-based HM fortifier (HMF; control) versus a novel HMF-containing lipids (including docosahexaenoic acid and arachidonic acid), higher protein and lower carbohydrate levels (test). Our hypothesis was that weight growth velocity in the test group would be non-inferior to that in the control group.
Design: Double-blind, randomised controlled trial.
Setting: Nine European neonatal intensive care units.
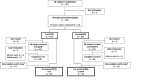
Patients: HM-fed infants born at <32-week gestational age.
Interventions: Fortification of HM with Test or Control HMF for a minimum of 21 days.
Primary outcome: Weight growth velocity between baseline and intervention day 21.
Results: From March 2018 to July 2020, 102 and 103 infants were enrolled in the test and control groups, respectively. Weight growth velocity during the first 21 days in the test group (mean 18.4 g/kg/day) was non-inferior to that of controls (mean 18.5 g/kg/day), with a difference in estimated means of -0.175 g/kg/day (90% CI -1.34 to +0.99 g/kg/day; per-protocol population). No significant differences between groups were observed for gain in length, head circumference or anthropometric Z-scores. Rates of digestive intolerance, stool frequency and consistency were comparable. No significant differences were reported in common neonatal morbidities including necrotising enterocolitis (test: 2.9%, control: 6.9%, mean difference -4.0% (95% CI -11.1% to 2.2%); all subjects treated population).
Conclusions: Use of the novel HMF containing lipids, higher protein and lower carbohydrate levels supports adequate postnatal growth and appears safe and well tolerated in very preterm infants.
Trial registration number: NCT03315221.
Keywords: Growth; Intensive Care Units, Neonatal; Neonatology.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: EvdH, AG and AB are employees of Danone Nutricia Research, Utrecht, The Netherlands. PC declares unrestricted research funding paid to his employing institution (NNUHFT) by Danone Early Life Nutrition, and conference travel and accommodation reimbursements received from Nutricia and Nestle in 2018–19. The remaining authors have no conflicts of interest relevant for this study to declare.
Figures
References
-
- Picaud JC, Vincent M, Buffin R. In: Nutritional care of preterm infants scientific basis and practical guidelines. Koletzko B, Cheah F-C, Domellöf M, et al., editors. Vol. 122. Basel, Switzerland: Karger; 2021. Human milk fortification for preterm infants: a review; pp. 225–47.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
