Deciphering the largest disease-associated transcript isoforms in the human neural retina with advanced long-read sequencing approaches
- PMID: 40037841
- PMCID: PMC12047242
- DOI: 10.1101/gr.280060.124
Deciphering the largest disease-associated transcript isoforms in the human neural retina with advanced long-read sequencing approaches
Abstract
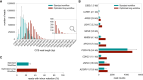
Sequencing technologies have long limited the comprehensive investigation of large transcripts associated with inherited retinal diseases (IRDs) like Usher syndrome, which involves 11 associated genes with transcripts up to 19.6 kb. To address this, we used PacBio long-read mRNA isoform sequencing (Iso-Seq) following standard library preparation and an optimized workflow to enrich for long transcripts in the human neural retina. While our workflow achieved sequencing of transcripts up to 15 kb, this was insufficient for Usher syndrome-associated genes USH2A and ADGRV1, with transcripts of 18.9 kb and 19.6 kb, respectively. To overcome this, we employed the Samplix Xdrop System for indirect target enrichment of cDNA, a technique typically used for genomic DNA capture. This method facilitated the successful capture and sequencing of ADGRV1 transcripts as well as full-length 18.9 kb USH2A transcripts. By combining algorithmic analysis with detailed manual curation of sequenced reads, we identified novel isoforms characterized by an alternative 5' transcription start site, the inclusion of previously unannotated exons, or alternative splicing events across the 11 Usher syndrome-associated genes. These findings have significant implications for genetic diagnostics and therapeutic development. The analysis applied here on Usher syndrome-associated transcripts exemplifies a valuable approach that can be extended to explore the transcriptomic complexity of other IRD-associated genes in the complete transcriptome data set generated within this study. Additionally, we demonstrate the adaptability of the Samplix Xdrop System for capturing cDNA, and the optimized methodologies described can be expanded to facilitate the enrichment of large transcripts from various tissues of interest.
© 2025 Stemerdink et al.; Published by Cold Spring Harbor Laboratory Press.
Figures





References
-
- Adato A, Vreugde S, Joensuu T, Avidan N, Hamalainen R, Belenkiy O, Olender T, Bonne-Tamir B, Ben-Asher E, Espinos C, et al. 2002. USH3A transcripts encode clarin-1, a four-transmembrane-domain protein with a possible role in sensory synapses. Eur J Hum Genet 10: 339–350. 10.1038/sj.ejhg.5200831 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
