Mono and combination therapies in pulmonary arterial hypertension patients with comorbidities: A COMPERA analysis
- PMID: 40037846
- PMCID: PMC12287794
- DOI: 10.1002/ehf2.15254
Mono and combination therapies in pulmonary arterial hypertension patients with comorbidities: A COMPERA analysis
Abstract
Aims: Pulmonary arterial hypertension (PAH) is often diagnosed in elderly patients with comorbidities. Although initial monotherapy is recommended for these patients, the value of combination therapy remains unclear. Here, we compare the efficacy of initial monotherapy and combination therapy in PAH patients with cardiovascular comorbidities.
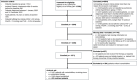
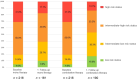
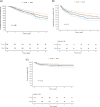
Methods and results: Data from adult patients with incident pre-capillary PAH and cardiovascular comorbidities from the COMPERA database (European registry for PH) were analysed. A matched-pair analysis of patients treated with monotherapy versus combination therapy based on age, sex, WHO functional class (FC) and 4-strata risk at baseline was performed. The matching strategy identified 216 pairs of PAH patients with cardiovascular comorbidities, who differed considerably from the enrolled patient population (n = 1871), especially in terms of mean age (mono: matched pairs 62.9 ± 13.5 years vs. 70.6 ± 11.4 years, combination: matched pairs 62.0 ± 13.6 years vs. 60.5 ± 14.9 years). In the matched-pair analysis, the initial combination therapy group showed more pronounced improvements in WHO-FC, N-terminal pro-B-type natriuretic peptide (BNP/NT-proBNP) and risk status than patients treated with initial monotherapy, with no significant differences in 6-min walk distance (6MWD), PAH-related hospitalisations, survival and drug discontinuation.
Conclusions: This analysis suggests that PAH patients with comorbidities may benefit more pronounced from combination therapy regarding WHO-FC, BNP/NT-pro-BNP and risk status without a significant difference in survival. Good tolerability is indicated. However, given the relatively younger patient matched subgroup, these findings may not necessarily apply to older patients with a wider range of comorbidities.
Keywords: COMPERA database; PAH and comorbidities; initial monotherapy and combination therapy; matched‐pair analysis.
© 2025 The Author(s). ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
Dirk Skowasch received fees for lectures and/or consulting and/or research support to institution from AstraZeneca, Boehringer Ingelheim, Chiesi, GSK, Janssen Pharmaceutical Companies of Johnson & Johnson, MSD, Sanofi, Pfizer, BMBF and DFG. Doerte Huscher none declared. Christine Pausch none declared. David Pittrow received fees for consultations from Actelion, Amgen, Aspen, Bayer, Biogen, Boehringer Ingelheim, Daiichi Sankyo, MSD, Novartis, Sanofi‐Genzyme, Takeda, Viatris and Zambon. Judith Wede is currently employed as Scientific Manager Therapeutic Area PAH at Janssen‐Cilag GmbH, a Johnson & Johnson company. Fabian Kreimendahl is currently employed as Scientific Specialist Medical Evidence Generation at Janssen‐Cilag GmbH, a Johnson & Johnson company. Stephan Rosenkranz has received fees for lectures and/or consultations from Abbott, Acceleron, Actelion, Bayer, Bristol‐Myers Squibb, Gilead, GlaxoSmithKline, Janssen, MSD, Novartis, Pfizer, United Therapeutics and Vifor; research grants to the institution from AstraZeneca, Actelion, Bayer Janssen and Novartis. Stephan Beckmann has received speaker fees from AstraZeneca and travel support from Janssen Cilag. Matthias Held has received speaker fees and honoraria for consultations from Actelion, Bayer, Boehringer Ingelheim Pharma, GlaxoSmithKline, Janssen, MSD, Novartis, Pfizer, Nycomed, Roche and Servier. Ekkehard Grünig served as speaker and/or consultant honoraria for Bayer Healthcare, Ferrer, GEBRO, GlaxoSmithKline, Janssen Biotech, Merck Sharp & Dohme (MSD) and OMT outside the submitted work. H. Ardeschir Ghofrani has received honoraria for consultations and/or speaking at conferences from Bayer HealthCare AG, Actelion, Encysive, Pfizer, Ergonex, Eli Lilly and Novartis; is a member of advisory boards for Acceleron, Bayer HealthCare AG, Pfizer, GlaxoSmithKline, Actelion, Eli Lilly, Merck, Encysive and Ergonex and has received governmental grants from the German Research Foundation, Excellence Cluster Cardiopulmonary Research, State Government of Hessen and the German Ministry for Education and Research. Hans Klose has received speaker fees and honoraria for consultations from Actelion, Bayer, GlaxoSmithKline, Janssen, MSD, Novartis, Pfizer and United Therapeutics. Andris Skride served as speaker and/or consultant for KRKA, Gossamer Bio, AOP Orphan and Novartis. Michael Halank received fees for lectures and/or consulting from AOP, AstraZeneca, Janssen Pharmaceutical Companies of Johnson & Johnson and MSD. Stefan Stadler received fees for lectures and/or consulting and/or research support to institution from AOP Health, Bayer, Gossamer Bio, Janssen‐Cilag GmbH, Keros Therapeutics, MSD and Pfizer. Marion Delcroix has received an institutional research grant from Janssen; institutional speaker and consultant fees from Bayer, MSD, Acceleron, AOP, Ferrer, Daiichi Sankyo, Inari, Janssen and Pfizer outside the submitted work and is holder of the Janssen Chair for Pulmonary Hypertension at KU Leuven. Anton Vonk‐Noordegraaf is supported by the Netherlands CardioVascular Research Initiative (CVON‐2012–08 PHAEDRA, CVON‐2017–10 DOLPHIN‐GENESIS) and the Netherlands Organization for Scientific Research (NWO‐VICI: 918.16.610). In addition his institute received speakers money from Johnson & Johnson, MSD, Actelion, Bayer and Ferrer in the past 3 years. Finally he served as a member of the scientific advisory board of Morphogen‐X, Ferrer, Gosammer Bio Services Inc, Altavant, MSD and Johnson & Johnson. Ralf Ewert received fees for lectures and/or consulting from LungPacer, OMT, AOP Orphan, AstraZeneca, Boehringer Ingelheim, Janssen Pharmaceutical and Berlin Chemie. Grzegorz Kopec served as consultant or speaker for Acceleron Pharma, Janssen, AOP Orphan, GossamerBio, Pfizer and Merck (paid to self). Marius M. Hoeper served as consultant or speaker for Acceleron Pharma, Inc., Actelion Pharmaceuticals, AOP Orphan, Bayer Healthcare, Ferrer, GossamerBio, Janssen Global Services, LLC and Merck & Co., Inc. (Rahway, NJ, USA) (paid to self). Karen M. Olsson served as consultant or speaker for Acceleron Pharma, Inc., Actelion Pharmaceuticals, AOP Orphan, Bayer Healthcare, Ferrer, GossamerBio, Janssen Global Services, LLC and Merck & Co., Inc. (Rahway, NJ, USA) (paid to self).
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

