Circulating mitochondrial DNA promotes M2 polarization of tumor associated macrophages and HCC resistance to sorafenib
- PMID: 40038250
- PMCID: PMC11880550
- DOI: 10.1038/s41419-025-07473-8
Circulating mitochondrial DNA promotes M2 polarization of tumor associated macrophages and HCC resistance to sorafenib
Abstract
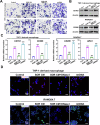
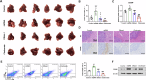
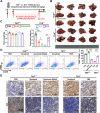
Mitochondrial damage-associated molecular patterns (DAMPs) including mitochondrial DNA (mtDNA), TFAM (transcription factor A, mitochondrial), and ATP, which play crucial roles in the regulation of inflammatory environment in human diseases. However, the role of mitochondrial DAMPs in regulating tumor microenvironment (TME) remains unclear. Herein, we demonstrate that infiltration of M2-type tumor-associated macrophages (TAMs) was correlated with the resistance of hepatocellular carcinoma (HCC) to sorafenib. We found that cell-free mtDNA in the plasma was significantly increased in sorafenib-resistant HCC mice. Sorafenib induced mitochondrial dysfunction and promoted the release of mtDNA into extracellular matrix of HCC. Macrophages retook the mtDNA in the TME of HCC, activated TLR9 signaling, and promoted the activation of NF-κB and the polarization of TAMs into M2. Application of DNase I to digest mtDNA or depletion of macrophages with clodronate liposomes reduced M2 macrophage infiltration, decreased the growth of HCC, and sensitized the tumors to sorafenib. Furthermore, we showed that blocking the activation of TLR9 enhanced the therapeutic effect of sorafenib in HCC. Together, we demonstrate that sorafenib treatment leads to the release of mtDNA into TME in HCC, which in turn facilitates the polarization of TAMs into M2 macrophages through TLR9 activation and aggravates the resistance of HCC to sorafenib. Our study reveals a novel mechanism underlying circulating mtDAMPs in remodeling the HCC microenvironment by reprograming the TAMs and provides a new strategy for improving the therapeutic effect of sorafenib and overcoming its resistance in HCC.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: All authors declare no competing interests. Ethics approval and consent to participate: This study was approved by the Ethics Committee of Henan University, and the informed consents were signed by all participants. Animal study was approved by the Institutional Animal Care and Use Committee of Henan University. We confirmed that all methods were performed in accordance with the relevant guidelines and regulations of the Ethics Committee of Henan University and Institutional Animal Care and Use Committee of Henan University. Consent for publication: Consent to publish has been obtained from all authors.
Figures






References
-
- Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. - PubMed
-
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. - PubMed
-
- Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, et al. Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 2022;19:151–72. - PubMed
-
- Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400:1345–62. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

