LNP-RNA-mediated antigen presentation leverages SARS-CoV-2-specific immunity for cancer treatment
- PMID: 40038251
- PMCID: PMC11880362
- DOI: 10.1038/s41467-025-57149-2
LNP-RNA-mediated antigen presentation leverages SARS-CoV-2-specific immunity for cancer treatment
Abstract
Lipid nanoparticle (LNP)-mRNA vaccines have demonstrated protective capability in combating SARS-CoV-2. Their extensive deployment across the global population leads to the broad presence of T-cell immunity against the SARS-CoV-2 spike protein, presenting an opportunity to harness this immunological response as a universal antigen target for cancer treatment. Herein, we design and synthesize a series of amino alcohol- or amino acid-derived ionizable lipids (AA lipids) and develop an LNP-RNA-based antigen presentation platform to redirect spike-specific T-cell immunity against cancer in mouse models. First, in a prime-boost regimen, AA2 LNP encapsulating spike mRNA elicit stronger T-cell immunity against the spike epitopes compared to FDA-approved LNPs (ALC-0315 and SM-102), highlighting the superior delivery efficiency of AA2 LNP. Next, AA15V LNP efficiently delivers self-amplifying RNAs (saRNAs) encoding spike epitope-loaded single-chain trimer (sSE-SCT) MHC I molecules into tumor tissues, thereby inducing the presentation of spike epitopes. Our results show that a single intratumoral (i.t.) treatment of AA15V LNP-sSE-SCTs suppresses tumor growth and extends the survival of B16F10 melanoma and A20 lymphoma tumor-bearing mice vaccinated with AA2 LNP-spike mRNA. Additionally, AA15V LNP-sSE-SCTs enable SE-SCT expression in ex vivo human glioblastoma and lung cancer samples, suggesting its potential in clinical translation.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: Y.D. is a scientific advisor in Arbor Biotechnologies. Y.D. is a co-founder and holds equity in Immunanoengineering Therapeutics. D.J.I. and R.W. are scientific advisory board members and hold equity in Strand Therapeutics. The remaining authors declare no competing interests.
Figures

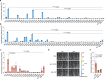
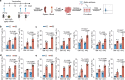
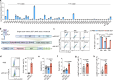


References
-
- Merad, M., Blish, C. A., Sallusto, F. & Iwasaki, A. The immunology and immunopathology of COVID-19. Science375, 1122–1127 (2022). - PubMed
-
- Krammer, F. SARS-CoV-2 vaccines in development. Nature586, 516–527 (2020). - PubMed
-
- Barbier, A. J., Jiang, A. Y., Zhang, P., Wooster, R. & Anderson, D. G. The clinical progress of mRNA vaccines and immunotherapies. Nat. Biotechnol.40, 840–854 (2022). - PubMed
MeSH terms
Substances
Grants and funding
- R35 GM119679/GM/NIGMS NIH HHS/United States
- R35 GM144117/GM/NIGMS NIH HHS/United States
- R35GM119679/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- R35GM144117/U.S. Department of Health & Human Services | NIH | National Institute of General Medical Sciences (NIGMS)
- R01 DK138025/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

