Safety, immunogenicity and effect on viral rebound of HTI vaccines combined with a TLR7 agonist in early-treated HIV-1 infection: a randomized, placebo-controlled phase 2a trial
- PMID: 40038256
- PMCID: PMC11880384
- DOI: 10.1038/s41467-025-57284-w
Safety, immunogenicity and effect on viral rebound of HTI vaccines combined with a TLR7 agonist in early-treated HIV-1 infection: a randomized, placebo-controlled phase 2a trial
Abstract
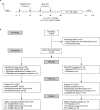
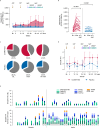
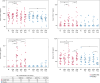
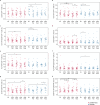
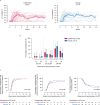
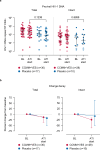
Building on results from the AELIX-002 trial with HIVACAT T-cell immunogen (HTI)-based vaccines, the AELIX-003 (NCT04364035) trial tested the safety of the combination of ChAdOx1.HTI (C) and MVA.HTI (M), with the TLR7 agonist vesatolimod (VES), in a double-blind, placebo-controlled, randomized clinical trial in 50 virally suppressed early-treated men with HIV-1 infection. Secondary objectives included immunogenicity and effects on viral rebound kinetics during a 24-week antiretroviral treatment interruption (ATI). The most common treatment-related adverse events were mild-to-moderate injection-site pain, influenza-like illness, headache, and fatigue. Strong, broad, and HTI-focused T-cell responses were induced by vaccination. All participants experienced viral rebound in ATI; 33.3% and 23.5% (P = 0.4494) of CCMM + VES and placebo recipients, respectively, remained off antiretroviral therapy for 24 weeks. Post hoc analysis confirmed a correlation between levels of HTI-specific T cells and prolonged time off antiretroviral therapy. The combination of HTI vaccines and VES was safe and elicited robust T-cell responses.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: C.Brander and B.M. are co-inventors of the HTI immunogen (patent application PCT/EP2013/051596). C.Brander, B.M., and I.M. are co-inventors of US patent Application No. 62/935,519 and US Appl. No. 62/851,546, which have relevance to the vaccine regimen used in this study. B.M. reports consultancy, advisory, and/or speaker fees from AELIX Therapeutics, Gilead Sciences, Janssen, ViiV, and MSD. J.M. reports advisory board and speaker fees and grant support from MSD, AbbVie, Boehringer Ingelheim, Gilead Sciences, ViiV Healthcare, Janssen-Cilag, and Bristol Myers Squibb. A.C. reports advisory and speaker fees and grant support from Gilead Sciences, Janssen, MSD, and ViiV Healthcare. P.S. reports advisory and/or speaker fees and/or support for attending meetings from Gilead Sciences, Janssen-Cilag, Merck Sharp & Dohme, Pfizer, and ViiV Healthcare, and has received a research grant from ViiV Healthcare, all outside of the submitted work. J.B. reports speaker fees and grant support from Gilead Sciences and MSD. J.C. reports speaker fees and grant support from Gilead Sciences and MSD. J.A. reports advisory board and speaker fees and grant support from Gilead Sciences, Janssen, MSD, ViiV; data and safety monitoring board membership with Grifols and HIPRA; and is an employee of the European AIDS Clinical Society (guidelines coordinator). A.I. reports advisory and speaker fees and grant support from Gilead Sciences, Janssen, MSD, Theratechnologies, and ViiV. S.M. reports speaking fees and research grants from Gilead Sciences, Janssen-Cilag, MSD, and ViiV. P.D. reports lecture and advisory board fees from ViiV Healthcare, MSD, GSK, Roche, Theratechnologies, Janssen & Cilag, and Gilead. Y.C., Y.T., R.G., D.S., D.L. J.J.W., S.G., and E.V. are employees and stockholders of Gilead Sciences, Inc. M.F. is an employee of ClinData Insight Inc, whose services were funded by Gilead Sciences, Inc. I.M. was an employee of AELIX Therapeutics, is currently an employee of Orion Biotechnology and is a consultant for Synklino. M.G.G. was an employee of AELIX Therapeutics at the time the research was conducted. J.M.M. has received consulting honoraria and/or research grants from Angelini, Contrafect, Cubist, Genentech, Gilead Sciences, Jansen, Lysovant, Medtronic, MSD, Novartis, Pfizer, and ViiV Healthcare, outside the submitted work. J.N. has received honoraria and/or speaking fees and/or financial support for attending conferences from AbbVie, Gilead Sciences, Janssen-Cilag, Merck Sharp & Dome, and ViiV Healthcare outside of the submitted work. C.Brander is the cofounder, shareholder, and CSO of AELIX. J.R.A. reports advisory and speaker fees and grant support from ViiV, Janssen, Gilead Sciences, MSD, and AELIX Therapeutics. The remaining authors declare that the research was conducted in the absence of any conflict of interest.
Figures


References
-
- Deeks, S. G. et al. Research priorities for an HIV cure: International AIDS Society Global Scientific Strategy 2021. Nat. Med.27, 2085–2098 (2021). - PubMed
-
- Bailón, L. et al. Safety, immunogenicity and effect on viral rebound of HTI vaccines in early treated HIV-1 infection: a randomized, placebo-controlled phase 1 trial. Nat. Med.28, 2611–2621 (2022). - PubMed
-
- Fidler, S. et al. Antiretroviral therapy alone versus antiretroviral therapy with a kick and kill approach, on measures of the HIV reservoir in participants with recent HIV infection (the RIVER trial): a phase 2, randomised trial. Lancet395, 888–898 (2020). - PubMed

