VHL ameliorates arecoline-induced oral submucosal fibrosis by promoting HDAC6 ubiquitination and blocking NF-κB pathway
- PMID: 40038412
- PMCID: PMC11880343
- DOI: 10.1038/s41598-025-91207-5
VHL ameliorates arecoline-induced oral submucosal fibrosis by promoting HDAC6 ubiquitination and blocking NF-κB pathway
Abstract
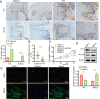
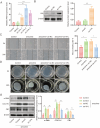
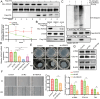
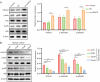
The chronic illness known as oral submucous fibrosis (OSF) results in tissue fibrosis, precancerous lesions, and scarring. It usually manifests itself in the buccal mucosa. It frequently occurs in the buccal mucosa. Von Hippel-Lindau (VHL) is an essential component of E3 ubiquitin ligase complex. The loss of VHL led to reduced fibrotic responses, accompanied by ameliorated fiber deposition. However, the precise impact of VHL on OSF is yet unclear. OSF tissues and normal mucosal tissues were applied to analyze the distinct expression of VHL and histone deacetylase 6 (HDAC6). Oral fibroblasts were treated to arecoline to simulate OSF in vitro, and molecular biological experiments were conducted to identify the role of VHL in buccal mucosa fibroblasts (BMFs). VHL was downregulated and HDAC6 was upregulated in OSF tissues and BMFs. Overexpression of VHL inhibited fibrosis in arecoline-treated BMFs. VHL inhibits the level of HDAC6 by inducing the ubiquitination of HDAC6. Knockdown of HDAC6 reduces the fibrogenic ability of BMFs. Furthermore, overexpression of HDAC6 contributes to the activation of NF-κB signaling in BMFs. HDAC6 selective inhibitor ACY-1215 inhibited the NF-κB signaling pathway. VHL attenuated arecoline-induced OSF by inhibiting the ubiquitination of HDAC6 and blocking NF-κB pathway. As a result, our study offers new perspectives into the discovery of novel tactics that can be employed against OSF.
Keywords: HDAC6; NF-κB; Oral submucosal fibrosis; Ubiquitination; Von Hippel-Lindau.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics statement: The Joint Ethics Committee of The First Affiliated Hospital of Hainan Medical University gave its approval for this study (2023-KYL-119 and HYLL-2023-437). Consent to participate: The informed consent has obtained.
Figures

Similar articles
-
Elevation of S100A4 expression in buccal mucosal fibroblasts by arecoline: involvement in the pathogenesis of oral submucous fibrosis.PLoS One. 2013;8(1):e55122. doi: 10.1371/journal.pone.0055122. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23383075 Free PMC article.
-
DNA methyltransferase 3A induces the occurrence of oral submucous fibrosis by promoting the methylation of the von Hippel-Lindau.Oral Dis. 2024 May;30(4):2325-2336. doi: 10.1111/odi.14725. Epub 2023 Sep 24. Oral Dis. 2024. PMID: 37743610
-
miR-200b ameliorates myofibroblast transdifferentiation in precancerous oral submucous fibrosis through targeting ZEB2.J Cell Mol Med. 2018 Sep;22(9):4130-4138. doi: 10.1111/jcmm.13690. Epub 2018 Jun 12. J Cell Mol Med. 2018. PMID: 29893466 Free PMC article.
-
Arecoline-induced myofibroblast transdifferentiation from human buccal mucosal fibroblasts is mediated by ZEB1.J Cell Mol Med. 2014 Apr;18(4):698-708. doi: 10.1111/jcmm.12219. Epub 2014 Jan 8. J Cell Mol Med. 2014. PMID: 24400868 Free PMC article.
-
Oral Submucous Fibrosis: A Review on Biomarkers, Pathogenic Mechanisms, and Treatments.Int J Mol Sci. 2020 Sep 30;21(19):7231. doi: 10.3390/ijms21197231. Int J Mol Sci. 2020. PMID: 33008091 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

