Abnormal arm swing movements in Parkinson's disease: onset, progression and response to L-Dopa
- PMID: 40038703
- PMCID: PMC11877831
- DOI: 10.1186/s12984-025-01589-w
Abnormal arm swing movements in Parkinson's disease: onset, progression and response to L-Dopa
Abstract
Background: Reduced arm swing movements during gait are an early motor manifestation of Parkinson's disease (PD). The clinical evolution, response to L-Dopa and pathophysiological underpinning of abnormal arm swing movements in PD remain largely unclear. By using a network of wearable sensors, this study objectively assesses arm swing movements during gait in PD patients across different disease stages and therapeutic conditions.
Methods: Twenty healthy subjects (HS) and 40 PD patients, including 20 early-stage and 20 mid-advanced subjects, underwent a 6-m Timed Up and Go test while monitored through a network of wearable inertial sensors. Arm swing movements were objectively evaluated in both hemibodies and different upper limb joints (shoulder and elbow), using specific time-domain (range of motion and velocity) and frequency-domain measures (harmonics and total harmonic distortion). To assess the effects of L-Dopa, patients under chronic dopaminergic therapy were randomly examined when OFF and ON therapy. Finally, clinical-behavioral correlations were investigated, primarily focusing on the relationship between arm swing movements and cardinal L-Dopa-responsive motor signs, including bradykinesia and rigidity.
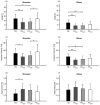
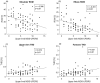
Results: Compared to HS, the whole group of PD patients showed reduced range of motion and velocity, alongside increased asymmetry of arm swing movements during gait. Additionally, a distinct increase in total harmonic distortion was found in patients. The kinematic changes were prominent in the early stage of the disease and progressively worsened owing to the involvement of the less affected hemibody. The time- and frequency-domain abnormalities were comparable in the two joints (i.e., shoulder and elbow). In the subgroup of patients under chronic dopaminergic treatment, L-Dopa restored patterns of arm swing movements. Finally, the kinematic alterations in arm swing movements during gait correlated with the clinical severity of bradykinesia and rigidity.
Conclusions: Arm swing movements during gait in PD are characterized by narrow, slow, and irregular patterns. As the disease progresses, arm swing movements deteriorate also in the less affected hemibody, without any joint specificity. The positive response to L-Dopa along with the significant correlation between kinematics and bradykinesia/rigidity scores points to the involvement of dopaminergic pathways in the pathophysiology of abnormal arm swing movements in PD.
Keywords: Arm swing; Bradykinesia; Parkinson’s disease; Rigidity; Wearable sensors.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: All participants provided written informed consent, and the study received approval from the institutional review board in compliance with the 1964 Declaration of Helsinki. Competing interests: The authors declare no competing interests.
Figures



References
-
- Navarro-López V, Fernández-Vázquez D, Molina-Rueda F, Cuesta-Gómez A, García-Prados P, Del-Valle-Gratacós M, et al. Arm-swing kinematics in Parkinson’s disease: A systematic review and meta-analysis. Gait Posture Ottobre. 2022;98:85–95. - PubMed
-
- Meyns P, Bruijn SM, Duysens J. The how and why of arm swing during human walking. Gait Posture Settembre. 2013;38(4):555–62. - PubMed
-
- Nieuwboer A, Weerdt WD, Dom R, Lesaffre E. A frequency and correlation analysis of motor deficits in Parkinson patients. Disabil Rehabil Gennaio. 1998;20(4):142–50. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

