Ibrutinib and acalabrutinib use and risk of incident atrial fibrillation: a propensity-matched analysis
- PMID: 40038806
- PMCID: PMC11877785
- DOI: 10.1186/s40164-025-00619-6
Ibrutinib and acalabrutinib use and risk of incident atrial fibrillation: a propensity-matched analysis
Abstract
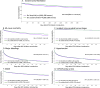
Ibrutinib and acalabrutinib are both associated with an increased risk of atrial fibrillation (AF); however, the comparative risk of AF between these 2 BTK inhibitors remains largely unknown. Our primary aim was to evaluate the risk of incident AF in patients exposed to ibrutinib compared to those exposed to acalabrutinib. Using the TriNetX research network database, we established a retrospective cohort of adult patients (≥ 18 years) previously diagnosed with a B-cell malignancy (using ICD-10-CM codes) in whom a first BTKi introduction occurred between January 1st, 2013 (first patient exposed to ibrutinib in TriNetX) and July 1st, 2024. Patients were divided into 2 groups based on their exposure to ibrutinib or acalabrutinib. After propensity score matching (PSM) across 37 covariates, Cox proportional hazard models were used to calculate the hazard ratios (HRs) and 95% confidence intervals (CIs) to compare the 2 matched groups. The appropriateness of the proportional hazard assumption was examined and risk differences (RDs) were used if appropriate. Results were summarized with the use of Kaplan-Meier survival curves. Follow-up started from 1 day after first BTKi introduction and continued over a 6-year follow-up period. A cohort of 12,449 patients exposed to ibrutinib and 4,131 to acalabrutinib were included in the study. After PSM, 4,090 patients remained in each group (1:1). During a mean duration of BTKi exposure of 2.3 ± 1.8 years, we found a significantly higher risk of incident AF in the ibrutinib group compared to the acalabrutinib group (RD 0.09, 95% CI 0.07-0.10). This difference was consistent across subgroups (age ≤ or > 75 and lower or higher baseline cardiovascular risk of developing AF). In conclusion, among patients with B-cell malignancies, the risk of developing incident AF is increased when treated with ibrutinib compared to acalabrutinib.Trial registration ClinicalTrial registration number: NCT06561243.
Keywords: Acalabrutinib; Atrial fibrillation; B-cell malignancies; Database; Ibrutinib.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

References
-
- Alexandre J, Salem J-E, Moslehi J, Sassier M, Ropert C, Cautela J, et al. Identification of anticancer drugs associated with atrial fibrillation: analysis of the WHO pharmacovigilance database. Eur Heart J Cardiovasc Pharmacother. 2021;7:312–20. - PubMed
-
- Font J, Milliez P, Ouazar A-B, Klok FA, Alexandre J. Atrial fibrillation, cancer and anticancer drugs. Arch Cardiovasc Dis. 2023;116:219–26. - PubMed
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

