A blood-based liquid biopsy analyzing soluble immune checkpoints and cytokines identifies distinct neuroendocrine tumors
- PMID: 40038821
- PMCID: PMC11881345
- DOI: 10.1186/s13046-025-03337-3
A blood-based liquid biopsy analyzing soluble immune checkpoints and cytokines identifies distinct neuroendocrine tumors
Abstract
Background: Neuroendocrine neoplasms (NENs) comprise a group of rare tumors originating from neuroendocrine cells, which are present in both endocrine glands and scattered throughout the body. Due to their scarcity and absence of specific markers, diagnosing NENs remains a complex challenge. Therefore, new biomarkers are required, ideally, in easy-to-obtain blood samples.
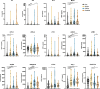
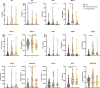
Methods: A panel of blood soluble immune checkpoints (sPD-L1, sPD-L2, sPD-1, sCD25, sTIM3, sLAG3, Galectin-9, sCD27, sB7.2 and sSIGLEC5) and cytokines (IL4, IL6, IP10 and MCP1) was quantified in a cohort of 139 NENs, including 29 pituitary NENs, 46 pheochromocytomas and paragangliomas, and 67 gastroenteropancreatic and pulmonary (GEPP) NENs, as well as in 64 healthy volunteers (HVs). The potential of these circulating immunological parameters to distinguish NENs from HVs, differentiate among various NENs subtypes, and predict their prognosis was evaluated using mathematical regression models. These immunological factors-based models generated scores that were evaluated by Receiver Operating Characteristic (ROC) and Area Under the Curve (AUC) analyses. Correlations between these scores and clinical data were performed. From these analyses, a minimal signature emerged, comprising the five shared immunological factors across the models: sCD25, sPD-L2, sTIM3, sLAG3, and Galectin-9. This refined signature was evaluated, validated, and checked for specificity against non-neuroendocrine tumors, demonstrating its potential as a clinically relevant tool for identifying distinct NENs.
Results: Most of the immunological factors analyzed showed specific expression patterns among different NENs. Scores based on signatures of these factors identified NENs with high efficiency, showing AUCs ranging between 0.948 and 0.993 depending on the comparison, and accuracies between 92.52% and 95.74%. These scores illustrated biological features of NENs including the similarity between pheochromocytomas and paragangliomas, the divergence between gastrointestinal and pulmonary NENs, and correlated with clinical features. Furthermore, the models demonstrated strong performance in distinguishing metastatic and exitus GEPP NENs, achieving sensitivities and specificities ranging from 80.95% to 88.89%. Additionally, an easy-to-implement minimal signature successfully identified all analyzed NENs with AUC values exceeding 0.900, and accuracies between 84.11% and 93.12%, which was internally validated by a discovery and validation randomization strategy. These findings highlight the effectiveness of the models and minimal signature in accurately diagnosing and differentiating NENs.
Conclusions: The analysis of soluble immunological factors in blood presents a promising liquid biopsy approach for identifying NENs, delivering critical insights for both prognosis and diagnosis. This study serves as a proof-of-concept for an innovative clinical tool that holds the potential to transform the management of these rare malignancies, providing a non-invasive and effective method for early detection and disease monitoring.
Keywords: Immunological factor; Liquid biopsy; Neuroendocrine neoplasm; Soluble immune checkpoint.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study involving human participants was reviewed and approved by local ethics committee of “La Paz University Hospital” with the reference number PI-5270. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures






References
-
- Gatta G, Capocaccia R, Botta L, Mallone S, De Angelis R, Ardanaz E, et al. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet—a population-based study. Lancet Oncol. 2017;18:1022–39. - PubMed
-
- Dasari A, Shen C, Halperin D, Zhao B, Zhou S, Xu Y, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335. Available from: http://oncology.jamanetwork.com/article.aspx?doi=10.1001/jamaoncol.2017..... - PMC - PubMed
-
- Ahmed M. Gastrointestinal neuroendocrine tumors in 2020. World J Gastrointest Oncol. 2020. p. 791–807. Available from: https://www.wjgnet.com/1948-5204/full/v12/i8/791.htm. - PMC - PubMed
-
- Gubbi S, Vijayvergia N, Yu JQ, Klubo-Gwiezdzinska J, Koch C. Immune checkpoint inhibitor therapy in neuroendocrine tumors. Horm Metab Res. 2022;54:795. Available from: https://www.thieme-connect.de/products/ejournals/abstract/10.1055/a-1908.... - DOI - PMC - PubMed
-
- Oronsky B, Ma PC, Morgensztern D, Carter CA. Nothing But NET: A Review of Neuroendocrine Tumors and Carcinomas. Neoplasia. 2017;19:991. Available from: https://www.sciencedirect.com/science/article/pii/S1476558617303470?via%.... - PMC - PubMed
MeSH terms
Substances
Grants and funding
- CP20/00106/Instituto de Salud Carlos III
- PI21/01178/Instituto de Salud Carlos III
- PIE 15/00065/Instituto de Salud Carlos III
- PI 18/00148/Instituto de Salud Carlos III
- PI 14/01234/Instituto de Salud Carlos III
- PI21/00869/Instituto de Salud Carlos III
- FORT23/00006/Instituto de Salud Carlos III
- CD21/00185/Instituto de Salud Carlos III
- PI20/01754/Instituto de Salud Carlos III
- PI24/01106/Instituto de Salud Carlos III
- IDEAS222745DELF/Fundación Científica Asociación Española Contra el Cáncer
- IND2022/BMD-23669/Comunidad de Madrid
- PEJ-2021-TL/BMD-21048/Comunidad de Madrid
- FPU2017-01317/Ministerio de Ciencia e Innovación
- PID2023-151388OB-I00/Ministerio de Ciencia, Innovación y Universidades
LinkOut - more resources
Full Text Sources
Research Materials

