Effectiveness and Safety of Upadacitinib Induction Therapy for 223 Patients With Crohn's Disease: A GETAID Multicentre Cohort Study
- PMID: 40038887
- PMCID: PMC12013792
- DOI: 10.1111/apt.70073
Effectiveness and Safety of Upadacitinib Induction Therapy for 223 Patients With Crohn's Disease: A GETAID Multicentre Cohort Study
Abstract
Background: Real-world effectiveness and safety of upadacitinib in patients with Crohn's disease (CD) remain unclear.
Aims: This study aimed to evaluate the effectiveness and safety of upadacitinib in a real-world cohort.
Methods: From September 2022 to June 2024, all consecutive patients with refractory luminal CD treated with once daily upadacitinib 45 mg in 29 French GETAID centres were retrospectively included. The primary outcome was steroid-free clinical remission (SFCR) at week 12, defined as a Harvey-Bradshaw Index (HBI) of < 4. Clinical response (decrease of ≥ 3 points in HBI and/or HBI < 4), clinical remission, biomarker remission, endoscopic and/or radiologic response and safety were also assessed.
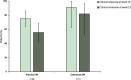
Results: Among the 223 patients included, all were previously exposed to at least one biologic (median 4, IQR [3, 4]) and 119 (53.8%) had prior intestinal resection. At week 12, SFCR was achieved in 107/197 (54%), clinical response in 129/197 (65%) and clinical remission in 111/197 (56%). A total of, 90 out of 173 (52%) achieved biomarker remission. Endoscopic and/or radiologic response was observed in 18/38 (47%) patients. Clinical response of extraintestinal manifestations was observed in 37/47 (79%) patients and clinical remission in 29/47 (62%). A total of, 65 adverse events (AEs) occurred in 58 patients (26%), including 17 serious AEs, 16 disease exacerbation and one case of colonic EBV-associated lymphoproliferative disorder. Acne was reported in 24/223 (11%) patients.
Conclusion: In this real-world cohort of highly refractory CD patients, upadacitinib induction resulted in a clinical response in about two-thirds of patients and in SFCR in half of the patients, with an acceptable safety profile.
Keywords: Crohn's disease; real‐world evidence; upadacitinib.
© 2025 The Author(s). Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
N.R. has received lecture/consultant fees from AbbVie, Ferring, Janssen and Takeda. A.A. received consulting fees from Abbvie, Lilly, MSD, Pfizer, Takeda, Tillotts Pharma, Adacyte, Janssen and Sandoz as well as lecture fees and travel accommodations from Abbvie, Lilly, Janssen, Pfizer, Takeda, Biogen, Fresenius Kabi, Adacyte, Amgen and Celltrion. P.S. received consulting fees from Takeda, Abbvie, Merck‐MSD, Biocodex, Janssen, Amgen, Astellas and Pfizer and grants from Biocodex and Janssen. R.A. has received lecture/consultant fees from AbbVie, Ferring, MSD, Celltrion, Biogen, Amgen, Sandoz, Pfizer, Alfasigma, Janssen and Takeda. D.L. declares counselling, boards, transports or fees from Abbvie, Amgen, Biogaran, Biogen, Celltrion, Ferring, Galapagos, Janssen, Lilly, Medac, MSD, Pfizer, Prometheus, Takeda and Theradiag. L.V. has received fees for lectures and/or consulting fees from Abbvie, Amgen, Johnson & Johnson, Celltrion, Takeda, Pfizer, Lilly, Ferring, MSD, Dr. Falk Pharma, Nordic Pharma, Alpha sigma. M.N. received board membership, consultancy or lecture fees from Abbvie, Amgen, Biogen, Celltrion, Ferring, Fresenius‐Kabi, Galapagos, Janssen, Lilly, Mayoli‐Spindler, MSD, Nordic Pharma, Pfizer, Takeda and Viatris. G.B. received lecture/consultant fees from Abbvie, Adacyte, Amgen, Celltrion, Edimark, Ferring, Fresinus Kabi, Galapagos, Janssen, Lilly, Pfizer, Sandoz, Takeda and Tillots. S.N. has received lecture/consultant fees from AbbVie, Ferring, Tillotts, Amgen, Fresenius, Sandoz, Pfizer, Celltrion, Gilead, Galapagos, Janssen and Takeda. C.G. has received lecture/consultant fees from AbbVie, AlfaSigma, Amgen, Celltrion, Janssen, Lilly, MSD, Pfizer and Takeda. C.R. has received lecture/consultant fees from Abbvie, Lilly, Janssen, Biogen, Amgen, Takeda and Galapagos. C.L.B. has served as a consultant for Abbvie, Celltrion, Janssen, Gilead and Takeda; has received payment for lectures from Abbvie, Amgen, Celltrion, Ferring, Fresenius Kabi, Galapagos, Janssen, Lilly, MSD, Nordic Pharma, Pfizer and Takeda; reports grant support from Abbvie and Takeda; has received meeting support fees from Abbvie, Celltrion, Ferring, Fresenius Kabi, Galapagos, Janssen, Lilly, MSD, Pfizer, Sandoz and Takeda. A.L.P. has received lecture/consultant fees from Janssen, Pfizer and Novartis. L.C. has received lecture/consultant fees from AbbVie, Celltrion, Biogen, Amgen, Sandoz, Pfizer, Alfasigma, Lilly, Janssen and Takeda. L.P.B. has received lecture/consultant fees from Abbvie, Abivax, Adacyte, Alimentiv, Amgen, Applied Molecular Transport, Arena, Banook, Biogen, BMS, Celltrion, Connect Biopharm, Cytoki Pharma, Enthera, Ferring, Fresenius Kabi, Galapagos, Genentech, Gilead, Gossamer Bio, GSK, IAC Image Analysis, Index Pharmaceuticals, Inotrem, Janssen, Lilly, Medac, Mopac, Morphic, MSD, Nordic Pharma, Novartis, Oncodesign Precision Medicine, ONO Pharma, OSE Immunotherapeuthics, Pandion Therapeuthics, Par’ Immune, Pfizer, Prometheus, Protagonist, Roche, Samsung, Sandoz, Sanofi, Satisfay, Takeda, Telavant, Theravance, Thermo Fischer, Tigenix, Tillots, Viatris, Vectivbio, Ventyx and Ysopia. L.P.B. has received grants from Celltrion, Fresenius Kabi, Medac, MSD and Takeda. M.F. has received lecture/consultant fees from AbbVie, Ferring, Tillotts, MSD, Biogen, Amgen, Fresenius, Hospira, Sandoz, Pfizer, Celgene, Gilead, Boehringer, Galapagos, Janssen and Takeda. N.R. has received lecture/consultant fees from AbbVie, Janssen and Takeda. The other authors state that they have no competing interests regarding this work to disclose.
Figures
References
-
- Singh S., Murad M. H., Fumery M., et al., “Comparative Efficacy and Safety of Biologic Therapies for Moderate‐To‐Severe Crohn's Disease: A Systematic Review and Network Meta‐Analysis,” Lancet Gastroenterology & Hepatology 6, no. 12 (2021): 1002–1014, 10.1016/S2468-1253(21)00312-5. - DOI - PMC - PubMed
-
- Turner D., Ricciuto A., Lewis A., et al., “STRIDE‐II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat‐To‐Target Strategies in IBD,” Gastroenterology 160, no. 5 (2021): 1570–1583, 10.1053/j.gastro.2020.12.031. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical