Prevalence of cytopenia(s) and somatic variants in patients with DDX41 mutant germline predisposition syndrome
- PMID: 40040251
- PMCID: PMC11985375
- DOI: 10.1111/bjh.20018
Prevalence of cytopenia(s) and somatic variants in patients with DDX41 mutant germline predisposition syndrome
Abstract
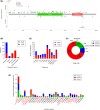
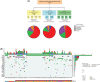
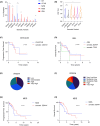
Germline variants in DDX41 (DDX41MT-germline predisposition syndrome [GPS]) are associated with predisposition to haematological malignancies (HM), including lymphoid and myeloid neoplasms (MN). We retrospectively analysed the clinical and molecular features of 195 patients diagnosed and treated at Mayo Clinic with DDX41MT-GPS. Patients with germline DDX41 pathogenic variants (42.3%) and variants of unknown significance (VUS, 57.6%) were included. The median age was 68.6 years (16.2-93.4). Ninety-two per cent were Caucasian, 64.1% were male and 30.8% had a family history of HM. There were 92 distinct germline variants among our cohort, and the most common was p.Met1? (15.9%), followed by p.Asp140Glyfs*2 (9.2%). Clinical diagnoses included asymptomatic carriers (10.2%), clonal cytopenia of undetermined significance (CCUS, 6.1%), myeloproliferative neoplasms (6.7%), myelodysplastic syndrome (40.5%), acute myeloid leukaemia (20.5%), lymphoid neoplasms (9.2%), plasma cell dyscrasias (6.1%) and solid tumours (22.5%). Patients with MN were older (median age 70 vs. 63.5 years) and more likely to be male (M:F ratio 2.3 vs. 1.0) and most patients (78.8%) with MN had a normal karyotype. The most common somatic variants involved DDX41 (34.4%), followed by TET2 (11.2%), DNMT3A (9.6%) and ASXL1 (9.2%). In summary, we have comprehensively described the spectrum of clinical phenotypes within the Mayo Clinic DDX41MT-GPS cohort.
Keywords: AML; CCUS; CHIP; MDS; germline DDX41; germline predisposition syndromes.
© 2025 The Author(s). British Journal of Haematology published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
MMP has served on advisory boards for CTI and has received research funding from StemLine, Kura, Epigenetix and Polaris. All other authors declare no relevant conflicts of interest in relation to the manuscript.
Figures
References
-
- Sebert M, Passet M, Raimbault A, Rahmé R, Raffoux E, Sicre de Fontbrune F, et al. Germline DDX41 mutations define a significant entity within adult MDS/AML patients. Blood. 2019;134(17):1441–1444. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

