Pharmacodynamics of Aspirin Through Gestation: Predictors of Aspirin Response and Association With Pregnancy Outcome, a Prospective Cohort Study
- PMID: 40040304
- PMCID: PMC11880114
- DOI: 10.1111/cts.70167
Pharmacodynamics of Aspirin Through Gestation: Predictors of Aspirin Response and Association With Pregnancy Outcome, a Prospective Cohort Study
Abstract
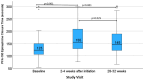
Low-dose aspirin is recommended for prevention of hypertensive disorders of pregnancy (HDP) and preterm birth (PTB) in high-risk pregnancies. There is limited data on factors impacting aspirin response in pregnancy. We aimed to evaluate predictors of aspirin response and association with pregnancy outcome with a prospective study of high-risk pregnancies taking 81 mg aspirin daily. Aspirin response was evaluated with Platelet Function Assay-100 (PFA-100) epinephrine closure time at baseline (< 16 weeks' gestation), follow-up 1 (2-4 weeks after aspirin initiation), and follow-up 2 (28-32 weeks gestation). Multivariable regression was used to identify factors associated with PFA-100 at each visit, and results presented with beta coefficient (B) and confidence interval. The median difference (MD) in PFA-100 in those with and without HDP or PTB was compared. Results included 108 who completed follow-up 1 and 96 who completed both visits with > 75% adherence. PFA-100 was increased from baseline at follow-ups 1 and 2 (MD 37 (27-49); MD 26 (15.5-38.5) respectively). At follow-up 1, obesity (B = -30 (-53 to -7) seconds), diabetes (B = -39 (-75 to -2) seconds), and age (B = 2.2 (0.3-4.0) seconds per year increased) were associated with PFA-100 response. Those with HDP in the current pregnancy versus not had similar aspirin response, but those with PTB versus term birth in the current pregnancy had reduced aspirin response at 28-32 weeks (MD -27 (-54 to -3) seconds). A daily dose of 81 mg aspirin results in platelet inhibition throughout gestation. Obesity, diabetes, and younger age are associated with reduced aspirin response in pregnancy.
Keywords: aspirin; pharmacodynamics; pharmacology; preeclampsia; pregnancy; preterm birth.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
RCB receives research grant funding from Covis Pharma, not related to this study. All other authors declare no conflicts of interests for this work.
Figures



References
-
- Henderson J. T., Vesco K., Senger C., Thomas R., and Redmond N., “Number 205 Aspirin Use to Prevent Preeclampsia and Related Morbidity and Mortality: An Evidence Update for the AHRQ Publication Number 21‐05274‐EF‐1” 2021. - PubMed
-
- van Vliet E. O. G., Askie L. A., Mol B. W. J., and Oudijk M. A., “Antiplatelet Agents and the Prevention of Spontaneous Preterm Birth: A Systematic Review and Meta‐Analysis,” Obstetrics and Gynecology 129, no. 2 (2017): 327–336. - PubMed
-
- Askie L. M., Duley L., Henderson‐Smart D. J., Stewart L. A., and PARIS Collaborative Group , “Antiplatelet Agents for Prevention of Pre‐Eclampsia: A Meta‐Analysis of Individual Patient Data,” Lancet 369, no. 9575 (2007): 1791–1798. - PubMed
-
- Banala C., Moreno S., Cruz Y., et al., “Impact of the ACOG Guideline Regarding Low Dose Aspirin for Prevention of Superimposed Preeclampsia in Women With Chronic Hypertension,” American Journal of Obstetrics and Gynecology 223, no. 3 (2020): 419.e1–419.e16, 10.1016/j.ajog.2020.03.004. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

