Paracetamol and its metabolites in children and adults with spinal muscular atrophy - a population pharmacokinetic model
- PMID: 40040359
- PMCID: PMC12199096
- DOI: 10.1002/bcp.70028
Paracetamol and its metabolites in children and adults with spinal muscular atrophy - a population pharmacokinetic model
Abstract
Aims: The aim of the study was to investigate whether differences in paracetamol pharmacokinetics (PK) between spinal muscular atrophy (SMA) patients and healthy controls (HC) could be attributed to specific clinical covariates.
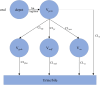
Methods: Nonlinear mixed-effects modelling (NONMEM 7.4) was used to develop a population PK model, explore covariates for paracetamol and its metabolites and perform simulations.
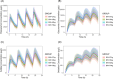
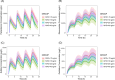
Results: With body weight as allometric scaling in the model, SMA disease resulted in a 58% (95% confidence interval [CI]: 20%-130%) increase in the volume of distribution for paracetamol and its metabolites compared to healthy controls. Decreased plasma myoglobin and plasma bilirubin concentrations, seen in SMA patients, resulted in a higher paracetamol leftover clearance (SMA, median: 13.30 L/h/70 kg, 95% CI: 9.14-18.29%; HC, median: 4.05 L/h/70 kg, 95% CI: 3.38-8.83%) and a shift from slower sulfate formation clearance (SMA, median: 8.78 L/h/70 kg, 95% CI: 7.22-9.61%; HC, median: 9.30 L/h/70 kg, 95% CI: 8.42-10.15%) and faster oxidative metabolites elimination clearance (SMA, median: 3.74 L/h/70 kg, 95% CI: 3.31-4.72%; HC, median: 3.25 L/h/70 kg, 95% CI: 2.87-3.92%). Simulations revealed that in SMA patients, higher bodyweight was associated with increased exposure to paracetamol and its metabolites.
Conclusions: The differences in PK between SMA patients and healthy controls could be explained by body weight and the disease itself. SMA patients should be dosed cautiously, ensuring doses do not exceed the recommended body weight adjusted limit.
Keywords: SMA; metabolites; paracetamol; pharmacokinetic.
© 2025 The Author(s). British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

