Sex Differences in the Efficacy of Glucagon-Like Peptide-1 Receptor Agonists for Weight Reduction: A Systematic Review and Meta-Analysis
- PMID: 40040445
- PMCID: PMC11880690
- DOI: 10.1111/1753-0407.70063
Sex Differences in the Efficacy of Glucagon-Like Peptide-1 Receptor Agonists for Weight Reduction: A Systematic Review and Meta-Analysis
Abstract
Aim: To verify sex differences of GLP-1RAs for weight reduction.
Methods: We searched RCTs reporting weight change by sex from PubMed, Web of Science, Embase, Cochrane Library, and ClinicalTrials registries. Meta-regression was performed to evaluate the association between weight reduction and sex differences. Subgroup analyses were stratified by individual GLP-1RA medications, dose, treatment duration, indication, type of control, background treatment, and baseline weight. The study protocol was registered (CRD42023480167).
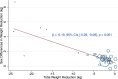
Results: Fourteen studies covering dulaglutide, exenatide, liraglutide, semaglutide, and retatrutide were included in this study. The meta-analysis showed that females lost more weight than males (MD 1.04 kg [95% CIs 0.70-1.38]; MD 1.69% [95% CI 0.78-2.61]). The pooled results of GLP-1RAs indicated similar results (MD 0.88 kg [95% CIs 0.67-1.09]). Meta-regression illustrated that substantial weight reduction was significantly relevant to greater gender differences (β = -0.19 [95% CIs -0.29 to -0.09]). Subgroup analysis demonstrated that indications for weight reduction increased the gender difference in weight reduction (MD 4.21 kg [95% CIs 1.75-6.67]). Background treatment, dose, duration of treatment, baseline weight, and type of control had no subgroup differences in the sex difference in weight reduction of GLP-1RAs. Dulaglutide (MD 0.88 kg [95% CIs 0.63-1.12]) and semaglutide (MD 1.04 kg [95% CIs 0.45-1.63]) showed statistically significant differences in weight reduction between males and females. No gender difference was observed in the exenatide subgroup analysis.
Conclusions: Females lost more weight than males when treated with GLP-1RAs for weight reduction. The sex difference in weight reduction became more pronounced as the degree of weight reduction increased. Indications for obesity could magnify this sex difference.
© 2025 The Author(s). Journal of Diabetes published by Ruijin Hospital, Shanghai JiaoTong University School of Medicine and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Sheng X., Ye X., Shi X., et al., “A Combination of Plasma Exchange and Steroids in the Treatment of a‐Lipoic Acid‐Induced Insulin Autoimmune Syndrome,” Endokrynologia Polska 72, no. 1 (2021): 81–82. - PubMed
-
- Quan H., Zhang H., Wei W., and Fang T., “Gender‐Related Different Effects of a Combined Therapy of Exenatide and Metformin on Overweight or Obesity Patients With Type 2 Diabetes Mellitus,” Journal of Diabetes and its Complications 30, no. 4 (2016): 686–692. - PubMed
-
- Ji L., Dong X., Li Y., et al., “Efficacy and Safety of Once‐Weekly Semaglutide Versus Once‐Daily Sitagliptin as Add‐On to Metformin in Patients With Type 2 Diabetes in SUSTAIN China: A 30‐Week, Double‐Blind, Phase 3a, Randomized Trial,” Diabetes, Obesity and Metabolism 23, no. 2 (2021): 404–414. - PMC - PubMed
-
- U.S. Food and Drug Administration , “Full Prescribing Information for Ozempic,” (2017).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

