Oral paltusotine, a nonpeptide selective somatostatin receptor 2 agonist: Mass balance, absolute bioavailability and metabolism in healthy participants
- PMID: 40040531
- PMCID: PMC12199097
- DOI: 10.1002/bcp.70020
Oral paltusotine, a nonpeptide selective somatostatin receptor 2 agonist: Mass balance, absolute bioavailability and metabolism in healthy participants
Abstract
Aims: Paltusotine is a novel, nonpeptide, selective somatostatin receptor 2 agonist in development for the treatment of acromegaly and carcinoid syndrome. This study investigated the mass balance, routes of excretion, absolute bioavailability and metabolite profile of orally administered paltusotine.
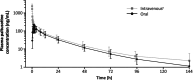
Methods: In Part A of the two-part, phase 1 study, a single dose (oral solution) of 20 mg paltusotine containing approximately 3.0 MBq (80 μCi) of 14C-labelled paltusotine was administered to six healthy male participants to evaluate the mass balance and metabolite profile of paltusotine. In Part B, a single dose (oral solution) of paltusotine 20 mg followed approximately 90 min later by a single microtracer intravenous bolus injection of approximately 50 μg paltusotine containing 0.0185 MBq (0.5 μCi) of 14C-labelled paltusotine was administered to five healthy male participants to assess absolute bioavailability of paltusotine.
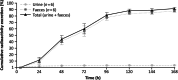
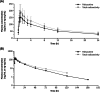
Results: The mean absolute bioavailability of paltusotine 20 mg was 69% (90% CI 59-82%). The mean recovery of total radioactivity was 94% (90% in faeces and 3.9% in urine), with excretion primarily via the biliary route. The exposure (AUC0-inf) ratio of unchanged paltusotine to total reactivity in plasma was 0.75, indicating that there were no abundant circulating metabolites. The geometric mean half-life (t1/2) for paltusotine in plasma was 26-28 h. Treatment-emergent adverse events associated with paltusotine were mild and transient.
Conclusions: Oral dosing with paltusotine is associated with efficient absorption from the gastrointestinal tract. Most of the administered dose is excreted unchanged. Pharmacokinetic properties of paltusotine are supportive of once-daily dosing.
Keywords: bioavailability; drug metabolism; endocrinology; phase I.
© 2025 The Author(s). British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
Rosa Luo, Deepak Dalvie, Lance Goulet, R. Scott Struthers and Alan S. Krasner are employees of Crinetics Pharmaceuticals. Ajay Madan and Christine T. Ferrara‐Cook were employees of Crinetics Pharmaceuticals at the time the study was conducted.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

