Treatment Patterns, Healthcare Resource Utilization, and Costs Among Patients Diagnosed With Locally Advanced/Metastatic Urothelial Carcinoma Prior to Receiving Enfortumab Vedotin: A Real-World Evidence Study
- PMID: 40040557
- PMCID: PMC12146246
- DOI: 10.1111/iju.70023
Treatment Patterns, Healthcare Resource Utilization, and Costs Among Patients Diagnosed With Locally Advanced/Metastatic Urothelial Carcinoma Prior to Receiving Enfortumab Vedotin: A Real-World Evidence Study
Abstract
Objective: To describe real-world patient characteristics, prior treatment patterns, and associated healthcare resource utilization (HRU) and costs among patients with locally advanced/metastatic urothelial carcinoma (la/mUC) treated with enfortumab vedotin (EV).
Methods: This retrospective study used the United States (US) Centers for Medicare and Medicaid Services 100% Medicare claims data from 2015 to 2020. Included patients had a diagnosis of la/mUC and received treatment with EV. The index date was the EV initiation date. Endpoints included HRU and costs 12 months before the index date (baseline period) and treatment patterns before EV initiation. Results were summarized descriptively using means and standard deviations for continuous variables, and frequency counts and percentages for categorical variables.
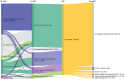
Results: Among the 529 included patients, the mean age at the time of EV initiation was 76.5 years. Most patients were White (88.1%) and male (77.1%). Common comorbidities were hypertension (85.1%), renal disease (65.2%), and peripheral vascular disease (42.5%). Platinum-based chemotherapy was the most frequent therapy two lines before EV initiation (43.9%). The most frequent therapy in the line before EV initiation was PD-1/L1 inhibitors (61.4%). The median duration of EV therapy was 4.1 months. The mean all-cause healthcare cost during the baseline period was $106 258 per patient, and 86% had at least one outpatient visit.
Conclusions: This real-world study demonstrated that most US patients with la/mUC received platinum-based chemotherapy or a PD-1/L1 inhibitor prior to EV therapy from 2015 to 2020. HRU and costs 12 months before EV initiation suggest a substantial burden in this population. Long-term studies with more recent data are warranted.
Keywords: enfortumab vedotin; healthcare costs; metastatic urothelial carcinoma; retrospective study; treatment patterns.
© 2025 The Author(s). International Journal of Urology published by John Wiley & Sons Australia, Ltd on behalf of The Japanese Urological Association.
Conflict of interest statement
A.K.M. reports consulting research collaborations with Astellas and Seagen, and consulting with Merck. L.M., C.Q., B.X., and C.Y. are employees of Astellas, the study sponsor. V.S. is an employee of Seagen, which was acquired by Pfizer in December 2023 and provided financial support for the conduct of this study. H.Y., Q.L., A.G., and A.L. are employees of Analysis Group Inc., which received financial support for the conduct of this study.
Figures


Similar articles
-
Real-world survival and economic burden among patients with locally advanced or metastatic urothelial carcinoma in the United States.Urol Oncol. 2025 Mar;43(3):189.e9-189.e18. doi: 10.1016/j.urolonc.2024.11.010. Epub 2024 Dec 14. Urol Oncol. 2025. PMID: 39675951
-
Real-world Effectiveness of Single-Agent Enfortumab Vedotin in Patients With Advanced Urothelial Carcinoma.Clin Genitourin Cancer. 2025 Feb;23(1):102287. doi: 10.1016/j.clgc.2024.102287. Epub 2024 Dec 6. Clin Genitourin Cancer. 2025. PMID: 39721833
-
Real-world burden of illness and unmet need in locally advanced or metastatic urothelial carcinoma following discontinuation of PD-1/L1 inhibitor therapy: A Medicare claims database analysis.Urol Oncol. 2021 Oct;39(10):733.e1-733.e10. doi: 10.1016/j.urolonc.2021.05.001. Epub 2021 Jul 5. Urol Oncol. 2021. PMID: 34238657
-
Epidemiology and treatment patterns for locally advanced or metastatic urothelial carcinoma: a systematic literature review and gap analysis.J Manag Care Spec Pharm. 2021 Feb;27(2):240-255. doi: 10.18553/jmcp.2020.20285. Epub 2020 Dec 23. J Manag Care Spec Pharm. 2021. PMID: 33355035 Free PMC article.
-
Enfortumab vedotin in the treatment of urothelial cancers and beyond.Future Oncol. 2022 Sep;18(27):3067-3084. doi: 10.2217/fon-2022-0328. Epub 2022 Aug 25. Future Oncol. 2022. PMID: 36004667 Review.
Cited by
-
Efficacy of modified enfortumab vedotin ineligible criteria (mEVITA) in advanced urothelial carcinoma.Sci Rep. 2025 Jul 18;15(1):26127. doi: 10.1038/s41598-025-09806-1. Sci Rep. 2025. PMID: 40681533 Free PMC article.
References
-
- “SEER Cancer Stat Facts: Female Breast Cancer,” 2021, https://seer.cancer.gov/statfacts/html/breast.html.
-
- Witjes J. A., Bruins H. M., Carrión A., et al., EAU Guidelines on Muscle‐Invasive and Metastatic Bladder Cancer (European Association of Urology, 2023).
-
- Flaig T. W., Spiess P. E., Abern M., et al., “NCCN Guidelines® Insights: Bladder Cancer, Version 3.2024,” Journal of the National Comprehensive Cancer Network 22, no. 4 (2024): 216–225. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

