Recognition of MR1-antigen complexes by TCR Vγ9Vδ2
- PMID: 40040716
- PMCID: PMC11876030
- DOI: 10.3389/fimmu.2025.1519128
Recognition of MR1-antigen complexes by TCR Vγ9Vδ2
Abstract
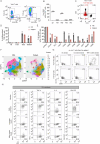
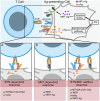
The TCR-mediated activation of T cells expressing the TCR Vγ9Vδ2 relies on an innate-like mechanism involving the butyrophilin 3A1, 3A2 and 2A1 molecules and phospho-antigens, without the participation of classical antigen-presenting molecules. Whether TCR Vγ9Vδ2 cells also recognize complexes composed of antigens and antigen-presenting molecules in an adaptive-like manner is unknown. Here, we identify MR1-autoreactive cells expressing the TCR Vγ9Vδ2. This MR1-restricted response is antigen- and CDR3δ-dependent and butyrophilin-independent. TCR gene transfer reconstitutes MR1-antigen recognition, and engineered TCR Vγ9Vδ2 tetramers interact with soluble MR1-antigen complexes in an antigen-dependent manner. These cells are present in healthy individuals with low frequency and are mostly CD8+ or CD4-CD8 double negative. We also describe a patient with autoimmune symptoms and TCR γδ lymphocytosis in which ~10% of circulating T cells are MR1-self-reactive and express a TCR Vγ9Vδ2. These cells release pro-inflammatory cytokines, suggesting a possible participation in disease pathogenesis. Thus, MR1-self-antigen complexes can interact with some TCRs Vγ9Vδ2, promoting full cell activation and potentially contributing to diseases.
Keywords: MR1; TCR γδ; Vγ9Vδ2; adaptive immunity; antigen recognition.
Copyright © 2025 Loureiro, Vacchini, Berloffa, Devan, Schaefer, Nosi, Colombo, Beshirova, Montanelli, Meyer, Sharpe, Chancellor, Recher, Mori and De Libero.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures







References
-
- Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. (1993) 178:1–16. doi: 10.1084/jem.178.1.1 - DOI - PMC - PubMed
-
- Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, et al. . An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. (1999) 189:1907–21. doi: 10.1084/jem.189.12.1907 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

