Advanced electrocatalysts for fuel cells: Evolution of active sites and synergistic properties of catalysts and carrier materials
- PMID: 40040831
- PMCID: PMC11875453
- DOI: 10.1002/EXP.20230052
Advanced electrocatalysts for fuel cells: Evolution of active sites and synergistic properties of catalysts and carrier materials
Abstract
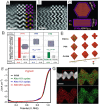
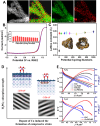
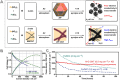
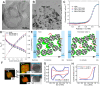
Proton exchange-membrane fuel cell (PEMFC) is a clean and efficient type of energy storage device. However, the sluggish reaction rate of the cathode oxygen reduction reaction (ORR) has been a significant problem in its development. This review reports the recent progress of advanced electrocatalysts focusing on the interface/surface electronic structure and exploring the synergistic relationship of precious-based and non-precious metal-based catalysts and support materials. The support materials contain non-metal (C/N/Si, etc.) and metal-based structures, which have demonstrated a crucial role in the synergistic enhancement of electrocatalytic properties, especially for high-temperature fuel cell systems. To improve the strong interaction, some exciting synergistic strategies by doping and coating heterogeneous elements or connecting polymeric ligands containing carbon and nitrogen were also shown herein. Besides the typical role of the crystal surface, phase structure, lattice strain, etc., the evolution of structure-performance relations was also highlighted in real-time tests. The advanced in situ characterization techniques were also reviewed to emphasize the accurate structure-performance relations. Finally, the challenge and prospect for developing the ORR electrocatalysts were concluded for commercial applications in low- and high-temperature fuel cell systems.
Keywords: active site; electrocatalyst; low/high‐temperature fuel cell; oxygen reduction reaction; synergistic property.
© 2024 The Author(s). Exploration published by Henan University and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Xiao F., Wang Y.‐C., Wu Z.‐P., Chen G., Yang F., Zhu S., Siddharth K., Kong Z., Lu A., Li J.‐C., Zhong C.‐J., Zhou Z.‐Y., Shao M., Adv. Mater. 2021, 33, 2006292. - PubMed
-
- Yang L., Shui J., Du L., Shao Y., Liu J., Dai L., Hu Z., Adv. Mater. 2019, 31, e1804799. - PubMed
-
- Shao Q., Wang P., Zhu T., Huang X., Acc. Chem. Res. 2019, 52, 3384. - PubMed
-
- Li W., Wang D., Zhang Y., Tao L., Wang T., Zou Y., Wang Y., Chen R., Wang S., Adv. Mater. 2020, 32, e1907879. - PubMed
-
- Huang L., Zaman S., Tian X., Wang Z., Fang W., Xia B. Y., Acc. Chem. Res. 2021, 54, 311. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
