Cost-effectiveness of an outreach program for HCC screening in patients with cirrhosis: a microsimulation modeling study
- PMID: 40040860
- PMCID: PMC11876903
- DOI: 10.1016/j.eclinm.2025.103113
Cost-effectiveness of an outreach program for HCC screening in patients with cirrhosis: a microsimulation modeling study
Abstract
Background: Patients with cirrhosis are at high risk for hepatocellular carcinoma (HCC), but few undergo guideline-recommended semi-annual screening. Randomized clinical trials (RCTs) demonstrate that mailed outreach can increase screening versus visit-based screening. We estimated the costs and cost-effectiveness of an outreach strategy versus usual care.
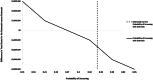
Methods: We built a 10-year Markov chain Monte Carlo microsimulation model to conduct a cost-effectiveness analysis comparing a mailed outreach program versus usual care for HCC screening in a cohort of 10,000 patients with cirrhosis. Model inputs were based on literature review (2005-current), and costs were based on inflation-adjusted estimates from Surveillance, Epidemiology, and End Results (SEER)-Medicare claims data. We conducted one-way sensitivity analyses for HCC incidence, outreach costs, efficacy of the outreach strategy to increase screening, and efficacy of curative (versus palliative) HCC treatments.
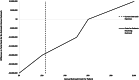
Findings: Mailed outreach was estimated to cost $32.45 per patient in the first year and $21.90 per patient in subsequent years. The outreach program increased the number of HCC patients detected at an early stage by 48.4% and increased quality-adjusted life years (QALYs) by 300. Cost savings from these increases offset the costs of mailed outreach. Mailed outreach remained cost-effective across a wide range of HCC incidence rates, outreach costs, efficacy of the outreach strategy to increase screening, and the efficacy of curative HCC treatments. Annual out-of-pocket patient costs in the outreach arm were low at $13 per year.
Interpretation: Mailed outreach to encourage HCC screening in patients with cirrhosis dominates usual care and should be considered for implementation in routine practice.
Funding: National Cancer Institute and Cancer Prevention Research Institute of Texas.
Keywords: Interventions; Liver cancer; Outreach; Screening; Ultrasound.
© 2025 The Author(s).
Conflict of interest statement
Amit G. Singal has served as a consultant or on advisory boards for Genentech, AztraZeneca, Eisai, Bayer, Exelixis, Elevar, Merck, Boston Scientific, Sirtex, HistoSonics, FujiFilm Medical Sciences, Exact Sciences, Roche, Glycotest, Abbott, Freenome, and GRAIL. None of the other authors have any relevant conflicts of interest.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources

