Evaluating digital adherence support in asthma: the ADITION non-interventional study
- PMID: 40040901
- PMCID: PMC11874225
- DOI: 10.1183/23120541.00734-2024
Evaluating digital adherence support in asthma: the ADITION non-interventional study
Abstract
Background: Poor adherence to asthma maintenance therapy is associated with worse outcomes. A solution could be digital adherence support. This study evaluated asthma control and adherence in patients using mometasone furoate/indacaterol/glycopyrronium (MF/IND/GLY) with a digital support system or using any inhaled corticosteroid/long-acting β2-agonist/long-acting muscarinic antagonist (ICS/LABA/LAMA) combination without support.
Methods: This prospective, non-interventional, multicentre, open-label study enrolled adults with asthma in Germany. Prior to inclusion, treatment was initiated with MF/IND/GLY with digital support or with any ICS/LABA/LAMA without digital support. The primary end-point was change in Asthma Control Test (ACT) at 6 months.
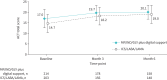
Results: Of 222 and 203 patients in the MF/IND/GLY plus digital support and ICS/LABA/LAMA groups, 76.1% and 74.9% completed follow-up, respectively. Baseline mean ACT total scores were 17.0 and 14.7, with mean changes from baseline at 6 months of 3.0 and 4.1, respectively; following propensity matching (n=92 per group), mean changes were similar in the two groups, with overlapping 95% confidence intervals (2.9 (95% CI 1.9-3.9) and 4.0 (95% CI 3.0-5.1), respectively). At enrolment, patients were overall moderately adherent to maintenance therapy, with limited changes over the study. The overall incidence of adverse events was similar in the two groups (29.5% and 27.3% of patients, respectively).
Conclusions: Patients using MF/IND/GLY with digital support had similar improvements in asthma control to those receiving ICS/LABA/LAMA alone, with minimal changes in adherence. These results illustrate the challenges in evaluating asthma control and adherence in non-interventional studies. Further studies are required to evaluate the value of digital support systems and how they can be used to optimise inhaler adherence.
Copyright ©The authors 2025.
Conflict of interest statement
Conflict of interest: In addition to the medical writing support disclosed in the Acknowledgements, the authors report the following conflicts of interest. H. Woehrle received research grants from Novartis and ResMed, consulting fees from AstraZeneca, Allergopharma, Bioprojet, Chiesi, GlaxoSmithKline, Novartis, Inspire, Jazz, ResMed and Teva, payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, Bioprojet, Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Novartis, Inspire, Jazz, ResMed and Sanofi, and is the head of the pulmonology working group in Baden-Wuerteemberg. All are outside the scope of the current manuscript. J. Driemert received consulting fees, payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events, payment for expert testimony, support for attending meetings and/or travel, participation on a data safety monitoring board or advisory board, and receipt of equipment, materials, drugs, medical writing, gifts or other services from Novartis, GlaxoSmithKline, Chiesi, Berlin-Chemie, Boehringer Ingelheim, Sanofi, AstraZeneca, Hexal and Bencard Allergie. All are outside the scope of the current manuscript. L. Jerrentrup received grants or contracts (to his research centre) for ongoing interventional and non-interventional studies from AstraZeneca, Genentech, Roche, Chiesi, GlaxoSmithKline, Bellus Health, BMS, Fortrea, Bayer, Insmed, MSD, Sterna Biologicals, Berlin-Chemie and Novartis. All are outside the scope of the current manuscript. C. Schiefer received consulting fees from Boehringer Ingelheim, AstraZeneca, Chiesi, Novartis and GlaxoSmithKline, payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from AstraZeneca, Berlin-Chemie and GlaxoSmithKline, payment for expert testimony from AstraZeneca, and participation on a data safety monitoring board or advisory board from Chiesi, GlaxoSmithKline and AstraZeneca. All are outside the scope of the current manuscript. G. Bushart was an employee of the sponsor, Novartis Pharma GmbH, at the time the study was conducted. I. Schwab Sauerbeck is an employee of the sponsor, Novartis Pharma GmbH.
Figures

References
-
- Jansen EM, van de Hei SJ, Dierick BJH, et al. Global burden of medication non-adherence in chronic obstructive pulmonary disease (COPD) and asthma: a narrative review of the clinical and economic case for smart inhalers. J Thorac Dis 2021; 13: 3846–3864. doi: 10.21037/jtd-20-2360 - DOI - PMC - PubMed
-
- Global Initiative for Asthma (GINA) . Global Strategy for Asthma Management and Prevention. 2024. Available from: http://ginasthma.org/
LinkOut - more resources
Full Text Sources
