Microfluidics for the biological analysis of atmospheric ice-nucleating particles: Perspectives and challenges
- PMID: 40041008
- PMCID: PMC11878220
- DOI: 10.1063/5.0236911
Microfluidics for the biological analysis of atmospheric ice-nucleating particles: Perspectives and challenges
Abstract
Atmospheric ice-nucleating particles (INPs) make up a vanishingly small proportion of atmospheric aerosol but are key to triggering the freezing of supercooled liquid water droplets, altering the lifetime and radiative properties of clouds and having a substantial impact on weather and climate. However, INPs are notoriously difficult to model due to a lack of information on their global sources, sinks, concentrations, and activity, necessitating the development of new instrumentation for quantifying and characterizing INPs in a rapid and automated manner. Microfluidic technology has been increasingly adopted by ice nucleation research groups in recent years as a means of performing droplet freezing analysis of INPs, enabling the measurement of hundreds or thousands of droplets per experiment at temperatures down to the homogeneous freezing of water. The potential for microfluidics extends far beyond this, with an entire toolbox of bioanalytical separation and detection techniques developed over 30 years for medical applications. Such methods could easily be adapted to biological and biogenic INP analysis to revolutionize the field, for example, in the identification and quantification of ice-nucleating bacteria and fungi. Combined with miniaturized sampling techniques, we can envisage the development and deployment of microfluidic sample-to-answer platforms for automated, user-friendly sampling and analysis of biological INPs in the field that would enable a greater understanding of their global and seasonal activity. Here, we review the various components that such a platform would incorporate to highlight the feasibility, and the challenges, of such an endeavor, from sampling and droplet freezing assays to separations and bioanalysis.
© 2025 Author(s).
Conflict of interest statement
The authors have no conflicts to disclose.
Figures

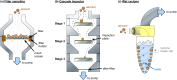


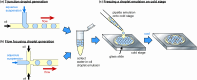

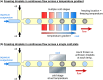

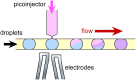
References
-
- Vali G., Bull. Am. Meteorol. Soc. 56(11), 1180–1184 (1975). 10.1175/1520-0477-56.11.1180 - DOI
-
- Jiusto J. E. and Lavoie R. L., Bull. Am. Meteorol. Soc. 56(11), 1175–1179 (1975). 10.1175/1520-0477-56.11.1175 - DOI
-
- Kanji Z. A., Ladino L. A., Wex H., Boose Y., Burkert-Kohn M., Cziczo D. J., and Krämer M., Meteorol. Monogr. 58, 1.1–1.33 (2017). 10.1175/AMSMONOGRAPHS-D-16-0006.1 - DOI
-
- Murray B. J., Carslaw K. S., and Field P. R., Atmos. Chem. Phys. 21(2), 665–679 (2021). 10.5194/acp-21-665-2021 - DOI
Publication types
LinkOut - more resources
Full Text Sources
