Liver transplantation for HBV-related liver disease: Impact of prophylaxis for HBV on HCC recurrence
- PMID: 40041120
- PMCID: PMC11876922
- DOI: 10.1016/j.jhepr.2024.101278
Liver transplantation for HBV-related liver disease: Impact of prophylaxis for HBV on HCC recurrence
Abstract
Background & aims: Conflicting data exist regarding optimal prophylaxis for HBV recurrence (HBV-R) after liver transplantation (LT), particularly in patients with hepatocellular carcinoma (HCC). We assessed current practices for HBV-R prophylaxis in Italy, evaluating rates, risk factors, and the clinical impact of HBV-R and HCC-R.
Methods: We performed a multicentric, retrospective study involving 20 Italian LT centers. All patients who underwent LT for HBV-related liver diseases between 2010 and 2021 were included. Logistic regression was used to identify predictors of HBV-R and HCC-R. Survival curves were estimated with the Kaplan-Meier method and compared with the log-rank test.
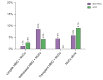
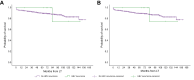
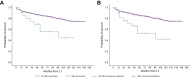
Results: We included 1,205 LT recipients (60.8% with HCC). HBV prophylaxis was prescribed in 99.7% of recipients, mostly with lifelong hepatitis B immunoglobulin+nucleos(t)ide analogues (HBIG+NUCs) (83.9%). Rates of HBV-R were 2.1% and 3.1% in patients transplanted without and with HCC, respectively. Median times from LT were 60 [9.5-77.5] and 5.5 [1-13] months, respectively. Recipients on lifelong HBIG+NUCs experienced lower rates of HBV-R than those in whom HBIG was withdrawn, used only during LT, or in those who received NUCs alone (2.3% vs. 6.2% vs. 1.9% vs. 8%, respectively; p = 0.042). In recipients with HCC, HCC-R rate was 10.8% (median time from LT: 18 months). At multivariate analysis, HBV-R (odds ratio [OR] 10.329; 95% CI 3.665-29.110), Child-Pugh C (OR 3.519; 95% CI 1.305-9.484), and microvascular invasion (OR 3.088; 95% CI 1.692-5.634) were independently associated with HCC-R. Five-year survival was lower in recipients who experienced HCC-R (32.5% vs. 92.4% in those who did not; p <0.001).
Conclusion: In Italy, HBV prophylaxis is mostly based on lifelong HBIG+NUCs. HBV-R was rare and not associated with survival in patients transplanted for decompensated cirrhosis. In patients transplanted for HCC, HBV-R was independently associated with HCC-R. The clinical implications of these findings deserve further investigation.
Impact and implications: In Italy, the combination of high-barrier nucleos(t)ide analogues and hepatitis B immunoglobulins remains the most widely used regimen for antiviral prophylaxis following liver transplantation for HBV-related liver disease. Hepatitis B recurrence after liver transplantation is a rare event and not associated with reduced survival. In transplant recipients with hepatocellular carcinoma, HBV recurrence was independently associated with hepatocellular carcinoma recurrence, though this may simply reflect an epiphenomenon without any causal relationship.
Keywords: HBV; HBV recurrence; HCC; HCC recurrence; HDV; liver transplantation.
© 2024 The Author(s).
Conflict of interest statement
SP: Consultant: Plasma Protein Therapeutics Association, Boehringer Ingelheim, Resolution Therapeutics; Speaking fees: Grifols, MEDSCAPE. PB has received lecture and consulting fees from Biotest, Chiesi Farmaceutici and Sandoz. The other authors have nothing to disclose. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures

References
-
- WHO - data from https://www.who.int/news-room/fact-sheets/detail/hepatitis-b 07/02/2024.
-
- Chen H.-Y., Shen D.-T., Ji D.-Z., et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut. 2019;68:512–521. - PubMed
LinkOut - more resources
Full Text Sources

