Application of microphysiological systems to unravel the mechanisms of schistosomiasis egg extravasation
- PMID: 40041145
- PMCID: PMC11876127
- DOI: 10.3389/fcimb.2025.1521265
Application of microphysiological systems to unravel the mechanisms of schistosomiasis egg extravasation
Abstract
Despite decades of control efforts, the prevalence of schistosomiasis remains high in many endemic regions, posing significant challenges to global health. One of the key factors contributing to the persistence of the disease is the complex life cycle of the Schistosoma parasite, the causative agent, which involves multiple stages of development and intricate interactions with its mammalian hosts and snails. Among the various stages of the parasite lifecycle, the deposition of eggs and their migration through host tissues is significant, as they initiate the onset of the disease pathology by inducing inflammatory reactions and tissue damage. However, our understanding of the mechanisms underlying Schistosoma egg extravasation remains limited, hindering efforts to develop effective interventions. Microphysiological systems, particularly organ-on-a-chip systems, offer a promising approach to study this phenomenon in a controlled experimental setting because they allow the replication of physiological microenvironments in vitro. This review provides an overview of schistosomiasis, introduces the concept of organ-on-a-chip technology, and discusses its potential applications in the field of schistosomiasis research.
Keywords: 3D microphysiological systems (3D MPS); animal models; egg extravasation; granuloma; organ-on-a-chip (OOC); schistosomiasis.
Copyright © 2025 Alfred, Ochola, Okeyo, Bae, Ogongo, Odongo, Njaanake and Robinson.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


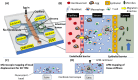

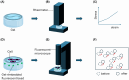
References
-
- Abdulla M. H., Ruelas D. S., Wolff B., Snedecor J., Lim K. C., Xu F., et al. . (2009). Drug discovery for schistosomiasis: Hit and lead compounds identified in a library of known drugs by medium-throughput phenotypic screening. PloS Negl. Trop. Dis. 3. doi: 10.1371/journal.pntd.0000478 - DOI - PMC - PubMed
-
- Abouel-Nour M. F., Lotfy M., El-Kady I., El-Shahat M., Doughty B. L. (2005). Localization of leucine aminopeptidase in the Schistosoma mansoni eggs and in liver tissue from infected mice. J. Egypt Soc. Parasitol. 35. - PubMed
-
- Abou Rashed A., Senna F. M., Sabry A. H., Morsy T. A. (1997). Studies on granuloma formation in Syrian hamsters experimentally infected with Schistosoma mansoni. J. Egypt Soc. Parasitol. 27. - PubMed

