Trametinib as a targeted treatment in cardiac and lymphatic presentations of Noonan syndrome
- PMID: 40041314
- PMCID: PMC11876372
- DOI: 10.3389/fped.2025.1475143
Trametinib as a targeted treatment in cardiac and lymphatic presentations of Noonan syndrome
Abstract
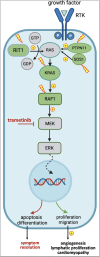
Introduction: Rare pathogenic variants in the PTPN11, KRAS, SOS1 and RAF1 genes are the main molecular causes of Noonan syndrome (NS). Most are dominant gain-of-function variants that cause an overactivation of the RAS/MAPK signaling pathway leading to uncontrolled cell proliferation in many organs and systems. Albeit phenotypically heterogeneous, NS can be associated with severe cardiovascular and lymphatic anomalies, potentially lethal during infancy, neonatal and fetal periods. MEK inhibitors, a class of drugs targeting the final steps of the RAS/MAPK pathway and originally developed for cancer therapy, have been tested in preclinical studies as a targeted treatment for NS. These studies led to the occasional off-label use of MEK inhibitors in patients with RASopathies.
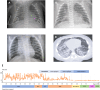
Methods: We report the case of a preterm infant with congenital pulmonary lymphangiectasis, chylothorax and hypoxic respiratory failure refractory to conventional management, who was treated with trametinib after identification of a NS PTPN11 class 5 variant. We performed a systematic review of the current published evidence on trametinib efficacy and safety for severe respiratory and/or cardiac manifestations in infants and children with Noonan syndrome, querying PubMed, Embase, Cochrane and Scopus databases, following the PRISMA guideline for systematic reviews, and using the Joanna Briggs Institute (JBI) Critical Appraisal tool for quality assessment of published evidence.
Results: In our patient, a five-week trametinib course, maximum dose 0.025 mg/kg/day, led to chylothorax resolution and gradual pulmonary function improvement, allowing extubation to non-invasive support, discharge home at a corrected age of 4 months, and weaning off home oxygen therapy by 10 months. No formal clinical trial of trametinib in neonatal/pediatric Noonan syndrome has been published to our knowledge. We collected 16 published cases, and added this case for reviewing trametinib regimen, efficacy and safety. A short-term improvement of symptoms was reported in all cases, with three deaths presumably unrelated to trametinib. Moderate side effects were reported in a subset of patients. Long-term follow-up data were not available.
Discussion: Trametinib is a promising drug in NS. Clinical trials are warranted to establish safety, efficacy, and standardized protocols for the use of trametinib as a rescue therapy in critically ill children and explore its potential place in the treatment of various NS comorbidities.
Systematic review registration: clinicaltrials.gov, identifier [NCT06555237].
Keywords: MEK inhibitors; Noonan syndrome; RASopathy; chylothorax; congenital pulmonary lymphangiectasia; hypertrophic cardiomyopathy; pulmonary valve stenosis; trametinib.
© 2025 De Brouchoven, Lorand, Bofferding, Sorlin, Van Damme and Danhaive.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor JH declared a shared parent affiliation with the authors at the time of review.
Figures

References
Publication types
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

