A Delphi Consensus Project to Capture Greek Experts' Opinion on the Position of Triple Therapies in COPD: Why, When and to Whom
- PMID: 40041472
- PMCID: PMC11878287
- DOI: 10.2147/COPD.S481337
A Delphi Consensus Project to Capture Greek Experts' Opinion on the Position of Triple Therapies in COPD: Why, When and to Whom
Abstract
Background: In recent years, COPD treatment has become more personalized considering specific patient's characteristics.
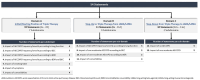
Aim and methods: We have performed a DELPHI consensus project to assess the level of consensus among Greek experts on the use of triple therapy in COPD as an initial and follow-up treatment. A three-round Delphi online survey was developed. The questionnaire was developed by a 6-member steering committee, included 54 statements, and divided into 3 domains: (A) triple therapy as initial treatment (divided into subdomains examining the impact of exacerbations based on lung function, bronchodilation reversibility and/or blood eosinophil count, smoking, symptoms, and comorbidities), (B) escalation to triple therapy from dual bronchodilation and (C) de-escalation from triple therapy to dual bronchodilation. The survey was funded by AstraZeneca and was hosted and analysed by an independent external company.
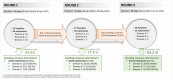
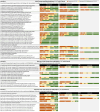
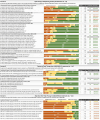
Results: Consensus was reached in 84.8%, 63% and 80% of statements for domains A, B and C, respectively. Experts agreed that initial treatment with triple therapy is a reasonable option for specific patients, while escalation from dual bronchodilation to triple therapy could be considered, besides frequent exacerbators, also in patients with a history of one moderate exacerbation, mainly in the presence of marked bronchodilator reversibility or high blood eosinophil count. Finally, there was a consensus that de-escalation from triple therapy to dual bronchodilation was inappropriate in patients who had experienced one moderate exacerbation in the previous year.
Conclusion: Although consensus was generated in several statements, panelists failed to reach consensus in many aspects of the use of triple therapy, identifying areas for further research.
Keywords: COPD; dual bronchodilation; exacerbations; inhaled corticosteroids; triple therapy.
© 2025 Papaioannou et al.
Conflict of interest statement
AIP has received honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, GSK, Menarini, Guidoti, Chiesi, ELPEN and Specialty Therapeutics. SL has received honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, GSK, Menarini, Guidoti, Chiesi, ELPEN and Specialty Therapeutics. TV has received honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, CHIESI, ELPEN, Innovis, GSK, Menarini, Novartis, and Pharmathen. NT has received honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, Boehringer Ingelheim, CHIESI, ELPEN, GSK, Menarini, Novartis, Pharmathen, Innovis, GILEAD, Guidotti, and Pfizer. KK has received grands from AstraZeneca, Boehringer Ingelheim, CHIESI, ELPEN, GSK, Menarini and Novartis Consulting fees from AstraZeneca, Boehringer Ingelheim, CHIESI, ELPEN, GSK, Menarini, Guidotti, Pfizer and Sanofi and honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Alector Pharmaceuticals, AstraZeneca, Boehringer Ingelheim, CHIESI, ELPEN, Gilead, GSK, Guidotti, Menarini, Pfizer and Sanofi, and he is a member of the GOLD assembly. GH has received honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from AstraZeneca, GSK, Menarini, Guidotti, Chiesi, ELPEN and Specialty Therapeutics. The authors report no other conflicts of interest in this work.
Figures


References
-
- Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2024; 2024. Available from: http://www.goldcopd.org. Accessed December 20, 2023.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

