Distinct MAIT cell phenotypes associated with sepsis clinical outcome in emergency department patients
- PMID: 40041474
- PMCID: PMC11879382
- DOI: 10.1002/cti2.70028
Distinct MAIT cell phenotypes associated with sepsis clinical outcome in emergency department patients
Abstract
Objectives: Rapid diagnosis and intervention are critical for sepsis patient outcomes. However, diagnosis is challenging because of a heterogenic patient group as well as sometimes vague symptoms when the patient presents at the emergency department. Mucosal-associated invariant T (MAIT) cells are rapid responders to infection, but their role and characteristics in the early course of sepsis remain unknown. Here, we evaluate the early MAIT cell characteristics in the blood of patients triggering a clinical sepsis alert system at the emergency department.
Methods: Peripheral blood mononuclear cells were isolated from freshly drawn blood and immediately stained. MAIT cell phenotyping analyses were conducted using multiparameter flow cytometry. All analyses were completed prior to the stratification of patients into sepsis or non-sepsis groups. Soluble factors in plasma were measured using a multiplex assay.
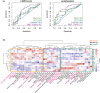
Results: Unsupervised high-dimensional phenotyping identified distinct MAIT cell activation profiles in sepsis and non-sepsis groups. Among sepsis patients, hierarchical clustering of MAIT cell phenotypes separated clinical endotypes into three groups with different infection focus, severity and aetiology. A prominent characteristic of sepsis severity was high expression of CD69 on MAIT cells, which was associated with organ dysfunction, lymphopenia and poor outcome. Plasma levels of IL-12, IL-15, TNF, IFNγ and CXCL10 correlated with the magnitude of MAIT cell activation in sepsis patients.
Conclusions: These clinical endotype-specific MAIT cell phenotypes presenting already in the emergency department are of interest for early patient identification and prognostication in sepsis.
Keywords: MAIT cells; immunophenotyping; sepsis; sepsis endotypes.
© 2025 The Author(s). Clinical & Translational Immunology published by John Wiley & Sons Australia, Ltd on behalf of Australian and New Zealand Society for Immunology, Inc.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity 2014; 40: 463–475. - PubMed
-
- Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med 2013; 369: 2063. - PubMed
-
- van der Poll T, Shankar‐Hari M, Wiersinga WJ. The immunology of sepsis. Immunity 2021; 54: 2450–2464. - PubMed
-
- van der Poll T, Opal SM. Host‐pathogen interactions in sepsis. Lancet Infect Dis 2008; 8: 32–43. - PubMed
LinkOut - more resources
Full Text Sources
