Successful application of dietary ketogenic metabolic therapy in patients with glioblastoma: a clinical study
- PMID: 40041752
- PMCID: PMC11876051
- DOI: 10.3389/fnut.2024.1489812
Successful application of dietary ketogenic metabolic therapy in patients with glioblastoma: a clinical study
Erratum in
-
Corrigendum: Successful application of dietary ketogenic metabolic therapy in patients with glioblastoma: a clinical study.Front Nutr. 2025 May 19;12:1614194. doi: 10.3389/fnut.2025.1614194. eCollection 2025. Front Nutr. 2025. PMID: 40458828 Free PMC article.
Abstract
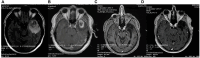
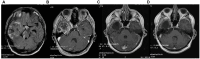
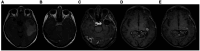
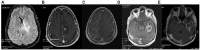
Introduction: Glioblastoma multiforme (GBM) ranks as one of the most aggressive primary malignant tumor affecting the brain. The persistent challenge of treatment failure and high relapse rates in GBM highlights the need for new treatment approaches. Recent research has pivoted toward exploring alternative therapeutic methods, such as the ketogenic diet, for GBM.
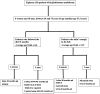
Methods: A total of 18 patients with GBM, 8 women and 10 men, aged between 34 and 75 years participated in a prospective study, examining the impact of ketogenic diet on tumor progression. The pool of patients originated from our hospital during the period from January 2016 until July 2021 and were followed until January 2024. As an assessment criterion, we set an optimistic target for adherence to the ketogenic diet beyond 6 months. We considered the therapeutic combination successful if the survival reached at least 3 years.
Results: Among the 18 patients participating in the study, 6 adhered to the ketogenic diet for more than 6 months. Of these patients, one patient passed away 43 months after diagnosis, achieving a survival of 3 years; another passed away at 36 months, narrowly missing the 3-year survival mark; and one is still alive at 33 months post-diagnosis but has yet to reach the 3-year milestone and is, therefore, not included in the final survival rate calculation. The remaining 3 are also still alive, completing 84,43 and 44 months of life, respectively. Consequently, the survival rate among these patients is 4 out of 6, or 66.7%. Of the 12 patients who did not adhere to the diet, only one reached 36 months of survival, while the rest have died in an average time of 15.7 ± 6.7 months, with a 3-year survival rate of 8.3%. Comparing the survival rates of the two groups, we see that the difference is 58.3% (66.7% versus 8.3%) and is statistically significant with p < 0.05 (0.0114) and X2 = 6.409.
Discussion: The outcomes observed in these patients offer promising insights into the potential benefits of the ketogenic diet on the progression of glioblastoma multiforme when compared to those who did not follow the diet consistently.
Keywords: brain; diet; glioblastoma; ketogenic; metabolic; multiforme; tumor.
Copyright © 2025 Kiryttopoulos, Evangeliou, Katsanika, Boukovinas, Foroglou, Zountsas, Cheva, Nikolopoulos, Zaramboukas, Duraj, Seyfried and Spilioti.
Conflict of interest statement
Author VN and TZ were employed by company Istodierevnitiki S.A. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



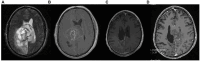
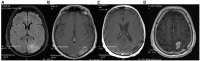
References
-
- Seyfried TN, Shivane AG, Kalamian M, Maroon JC, Mukherjee P, Zuccoli G. Ketogenic metabolic therapy, without chemo or radiation, for the long-term management of IDH1-mutant glioblastoma: an 80-month follow-up case report. Front Nutr. (2021) 8:682243. doi: 10.3389/fnut.2021.682243, PMID: - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

