Preclinical Evidence for the Use of Brexpiprazole + Antidepressant Treatment for Major Depressive Disorder and Post-Traumatic Stress Disorder: A Systematic Review
- PMID: 40041884
- PMCID: PMC11878111
- DOI: 10.2147/NDT.S501207
Preclinical Evidence for the Use of Brexpiprazole + Antidepressant Treatment for Major Depressive Disorder and Post-Traumatic Stress Disorder: A Systematic Review
Abstract
Purpose: Brexpiprazole, when administered with antidepressant therapy, may provide additional benefits due to complementary actions on noradrenaline (norepinephrine), serotonin, and dopamine neurotransmitter systems. This review addressed the question: what information can preclinical studies provide on the use of brexpiprazole + antidepressant treatment?
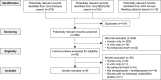
Methods: A systematic literature review was conducted to search for preclinical studies of brexpiprazole + antidepressant therapy that included a behavioral test relating to any psychiatric disorder. Ovid MEDLINE, Ovid Embase, and conference abstracts were searched (January 1, 2011-July 5, 2021). The statistically significant (p<0.05) findings for brexpiprazole + antidepressant were extracted.
Results: Of 296 records screened, nine articles were eligible, describing seven unique studies. In rodent models, including three models of depression (unpredictable chronic mild stress, social defeat stress, and lipopolysaccharide-induced depression), brexpiprazole + selective serotonin reuptake inhibitor (SSRI) or serotonin-noradrenaline reuptake inhibitor (SNRI) consistently showed statistically significant benefits over vehicle on depression-like behaviors (forced swim test, tail suspension test, sucrose preference), whereas brexpiprazole and antidepressant monotherapies did not. In the predator scent stress model of post-traumatic stress disorder (PTSD), brexpiprazole + SSRI (escitalopram) showed a significant benefit over vehicle and/or monotherapy on anxiety-like behaviors (elevated plus-maze) and hyperalertness (acoustic startle response), whereas brexpiprazole and escitalopram monotherapies did not significantly differ from vehicle. In the fear conditioning model of PTSD, brexpiprazole showed significant improvements whether administered as monotherapy or in combination with escitalopram.
Conclusion: Based on a small number of studies, the administration of brexpiprazole with an antidepressant appears to have a greater treatment effect than either brexpiprazole or antidepressant monotherapies in preclinical studies of depression- and PTSD-like behaviors. Thus, preclinical studies support evidence from randomized clinical trials for the therapeutic effects of adjunctive brexpiprazole in the treatment of major depressive disorder, and brexpiprazole in combination with sertraline in the treatment of PTSD. Funding: Otsuka/Lundbeck.
Keywords: PTSD; antidepressants; antipsychotics; behavior rating scale; depression.
Plain language summary
Studies of medications in animals can provide additional information to complement studies in humans. This review investigates what can be learned from animal studies of brexpiprazole (a medication known as an “atypical antipsychotic”) given together with an antidepressant medication. Seven animal studies that assess brexpiprazole + antidepressant treatment in rat or mouse models of depression and post-traumatic stress disorder were identified. Overall, these animal studies provide support for the observed benefits of brexpiprazole added to antidepressant treatment in major depressive disorder, and the ongoing development of brexpiprazole + antidepressant as a potential combination treatment for post-traumatic stress disorder.
© 2025 Brubaker et al.
Conflict of interest statement
Malaak Brubaker, Shivani Kapadia and Jessie S Chambers are full-time employees of Otsuka Pharmaceutical Development & Commercialization Inc. Vladimir Maletic was a consultant for AbbVie/Allergan, Acadia Pharmaceuticals, Inc., Alfasigma, USA, Inc., Alkermes, Inc., Biogen, Boehringer Ingelheim, Cerevel Therapeutics, LLC, Corium, H. Lundbeck A/S, Intra-Cellular Therapies, Ironshore, Janssen, Jazz Pharmaceuticals, LivaNova, Neurelis, Neumora, Noven Pharmaceuticals Inc, Otsuka America Pharmaceutical, Inc., Pax Medica, Relmada Therapeutics, Sage Pharmaceuticals, Sunovion Pharmaceuticals Inc., Supernus Pharmaceuticals, Inc., Takeda Pharmaceutical Company Limited, and Tris Pharma; and was part of a speakers bureau for AbbVie, Acadia, Alfasigma, Alkermes, Inc., Axsome, Corium, Eisai, Ironshore, Intra-Cellular, Janssen, H. Lundbeck A/S, Otsuka America Pharmaceutical, Inc., Sunovion, Supernus Pharmaceuticals Inc., and Takeda Pharmaceutical Company Limited. Christopher P Watling is an employee of Cambridge (a division of Prime), which received funding from Otsuka Pharmaceutical Development & Commercialization Inc. and Lundbeck LLC for this work. Leslie Citrome has acted as a consultant for AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, Biogen, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Cerevel, Clinilabs, COMPASS, Delpor, Eisai, Enteris BioPharma, HLS Therapeutics, Idorsia, INmune Bio, Impel, Intra-Cellular Therapies, Janssen, Karuna, Lundbeck, Luye, Lyndra, MapLight, Marvin, Medavante-ProPhase, Merck, Mitsubishi-Tanabe Pharma, Neumora, Neurocrine, Neurelis, Noema, Novartis, Noven, Otsuka, Ovid, Praxis, Recordati, Relmada, Reviva, Sage, Sumitomo/Sunovion, Supernus, Teva, University of Arizona, Vanda, and Wells Fargo, and has performed one-off ad hoc consulting for individuals/entities conducting marketing, commercial, or scientific scoping research; has acted as a speaker for AbbVie/Allergan, Acadia, Alkermes, Angelini, Axsome, BioXcel, Eisai, Idorsia, Intra-Cellular Therapies, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Recordati, Sage, Sunovion, Takeda, Teva, and CME activities organized by medical education companies such as Medscape, NACCME, NEI, Vindico, and universities and professional organizations/societies; owns stocks (small number of shares of common stock) in Bristol-Myers Squibb, Eli Lilly, J & J, Merck, Pfizer (purchased >10 years ago), and has stock options for Reviva; and has received royalties/publishing income from Taylor & Francis (Editor-in-Chief, Current Medical Research and Opinion, 2022–date), Wiley (Editor-in-Chief, International Journal of Clinical Practice, through end 2019), UpToDate (reviewer), Springer Healthcare (book), and Elsevier (Topic Editor, Psychiatry, Clinical Therapeutics). The authors report no other conflicts of interest in this work.
References
-
- Thase ME, Youakim JM, Skuban A, et al. Efficacy and safety of adjunctive brexpiprazole 2 mg in major depressive disorder: a Phase 3, randomized, placebo-controlled study in patients with inadequate response to antidepressants. J Clin Psychiatry. 2015;76(9):1224–1231. doi:10.4088/JCP.14m09688 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources