Free fatty acid receptor-4 regulates T-cell-mediated allogeneic reaction through activating an aryl hydrocarbon receptor pathway
- PMID: 40041898
- PMCID: PMC11873605
- DOI: 10.1016/j.apsb.2024.12.011
Free fatty acid receptor-4 regulates T-cell-mediated allogeneic reaction through activating an aryl hydrocarbon receptor pathway
Abstract
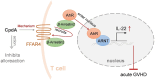
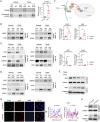
Targeting T-cell is a strategy to control allogeneic response disorders, such as acute graft-versus-host disease (GVHD) which is an important cause of therapy-failure after allogeneic hematopoietic cell transplants. Free fatty acid receptor-4 (FFAR4) is a regulator of obesity but its role in T-cell and allogeneic reactions is unknown. Here, we found knockout of Ffar4 in donor T-cells in a mouse allograft model increased acute GVHD whereas the natural FFAR4 ligands and the synthetic FFAR4 agonists decreased it. FFAR4 agonist-mediated anti-acute GVHD effects depended on FFAR4-expression in donor T-cells. The FFAR4 agonist CpdA suppressed donor T-cell-mediated alloreaction by activating an aryl hydrocarbon receptor (AhR) pathway. CpdA recruited β-Arrestin2 to FFAR4 which facilitated nuclear translocation of AhR and upregulation of IL-22. The CpdA-mediated anti-acute GVHD effect was absent in mice receiving Ahr-knockout or Il22-knockout T-cells. Recipient-expressing Ffar4 was also important for the anti-acute GVHD effect of CpdA which inhibited activation of antigen presenting cells. Importantly, CpdA decreased acute GVHD in obese mice, an effect also depended on Ffar4-expression in donor T-cells and recipients. Our study shows the immunoregulatory effect of FFAR4 in T-cell, and targeting FFAR4 might be a relative option for controlling allogeneic reactions in obese patients.
Keywords: Allogeneic reaction; Aryl hydrocarbon receptor; CpdA; Free fatty acid receptor-4; Graft-versus-host disease; Interleukin-22; Obesity; T-cell.
© 2025 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Penack O., Marchetti M., Ruutu T., Aljurf M., Bacigalupo A., Bonifazi F., et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:157–167. - PubMed
-
- Tan J.K., McKenzie C., Marino E., Macia L., Mackay C.R. Metabolite-sensing g protein-coupled receptors-facilitators of diet-related immune regulation. Annu Rev Immunol. 2017;35:371–402. - PubMed
-
- Duah M., Zhang K., Liang Y., Ayarick V.A., Xu K., Pan B. Immune regulation of poly unsaturated fatty acids and free fatty acid receptor 4. J Nutr Biochem. 2023;112 - PubMed
-
- Oh H., Loberiza F.R., Jr., Zhang M.J., Ringden O., Akiyama H., Asai T., et al. Comparison of graft-versus-host-disease and survival after HLA-identical sibling bone marrow transplantation in ethnic populations. Blood. 2005;105:1408–1416. - PubMed
LinkOut - more resources
Full Text Sources

