Ultra-Efficient and Selective Gold Separation via Second-Sphere Coordination of Aurous Dihalide Using a Nonporous Amorphous Superadsorbent
- PMID: 40042045
- PMCID: PMC12021077
- DOI: 10.1002/advs.202501397
Ultra-Efficient and Selective Gold Separation via Second-Sphere Coordination of Aurous Dihalide Using a Nonporous Amorphous Superadsorbent
Abstract
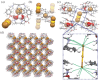
The escalating demand for gold, coupled with dwindling terrestrial reserves, underscores the urgent need for innovative separation strategies, including e-waste recycling and seawater extraction. However, the development of ultra-efficient, highly selective adsorbents capable of recovering trace amounts of gold from complex aquatic matrices remains a formidable challenge. Herein, a covalent organic superphane cage is reported as a nonporous amorphous superadsorbent (NAS) for selective and efficient gold recovery via intermolecular second-sphere coordination of AuBr₂⁻ (or AuCl₂⁻) ions, subsequently converted to metallic gold through disproportionation. NAS demonstrates outstanding performance, including an exceptional gold uptake capacity of 2750 mg g⁻¹, ultrafast adsorption kinetics (40 s), broad pH tolerance (1-11, up to 6 M acids), and remarkable gold uptake even in 36 wt.% HCl solution (821 mg g⁻¹). NAS achieves over 99% selective gold recovery, even amidst excess competing ions, retaining efficacy across 30 regeneration cycles. Its versatile and scalable design enables applications in gold separation from gold-bearing e-waste, catalytic residues, gold ores, and seawater. A large-scale trial recovered 23.8 Karat gold from printed circuit board leachates, positioning NAS as a sustainable and eco-friendly solution for industrial and environmental gold recovery.
Keywords: Covalent organic cage; Gold separation; Nonporous amorphous sorbent; Second‐sphere coordination; Superphane.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
Hunan University has applied one patent based on this work.
Figures




References
-
- Zhu B., Gong S., Cheng W., Chem. Soc. Rev. 2019, 48, 1668. - PubMed
-
- Syed S., Hydrometallurgy. 2012, 115–116, 30.
-
- Serpe A., in Waste Electrical and Electronic Equipment Recycling (Eds: Vegliò F., Birloaga I.), Woodhead Publishing, Sawston, UK: 2018, pp 271–332;
- b) Kerr R. A., Science. 2012, 335, 1038. - PubMed
-
- Diallo M. S., Kotte M. R., Cho M., Environ. Sci. Technol. 2015, 49, 9390. - PubMed
-
- Adams M. D., Gold Ore Processing: Project Development and Operations, 2nd ed., Elsevier, Amsterdam: 2016.
Grants and funding
LinkOut - more resources
Full Text Sources
