Single-Electron-Transfer-Mediated Carbonylation Reactions
- PMID: 40042084
- PMCID: PMC11924242
- DOI: 10.1021/acs.accounts.5c00039
Single-Electron-Transfer-Mediated Carbonylation Reactions
Abstract
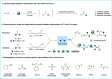
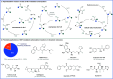
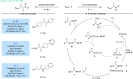
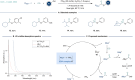
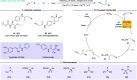
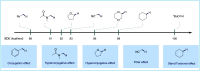
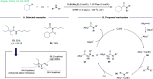
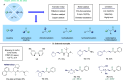
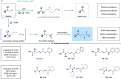
ConspectusTransition-metal-catalyzed carbonylation coupling methods have been accepted as an essential tool for producing carbonylated products over the past few decades. Despite its long-standing history and widespread industrial applications, several challenges remain in carbonylation chemistry. These include reliance on precious metal catalysts, the need of high-energy radiation, difficulties in carbonylation of unactivated chemical bonds, etc. As an alternative to classic two-electron transfer process, single-electron-transfer (SET)-mediated carbonylation has emerged as a powerful tool to achieve elusive carbonylation transformations. Over the past few years, carbonylation of commonly available functional handles, such as alkenes and alkyl halides, via the single-electron pathway has emerged as a valuable area of research.Our team has been dedicated to developing new carbonylation reactions using bulk chemicals to construct high-value carbonylated products. These reactions have broad synthetic and industrial applications, motivating us to explore SET-mediated carbonylation transformations for two key classes of bulk chemicals: alkanes and alkyl halides. Specifically, our work has centered on two main approaches: (1) Single-electron reduction of C(sp3)-X bonds: this strategy leverages single-electron reduction to activate C(sp3)-X bonds, promoting the formation of carbon radicals, which in turn promotes subsequent addition to metals or CO. However, a significant challenge lies in the highly negative reduction potential of certain substrates [Ered < -2 V compared to the saturated calomel electrode (SCE) for unactivated alkyl iodides]. Despite these challenges, the intrinsic reducibility of CO and the reactivity of various carbonyl-metal intermediates facilitate smooth reaction progress. (2) Single-electron oxidative of C(sp3)-H bonds: this strategy emphasizes efficiency, high atomic utilization, and minimal waste by bypassing traditional preactivation methods. Using 3d metal catalysts, we have successfully performed aminocarbonylation and alkoxycarbonylation on a wide range of C(sp3)-H bonds (such as those in aliphatic alkanes, ethers, amines, etc.). The above two approaches also enabled radical relay carbonylation of alkenes, allowing precise control over reaction intermediates and pathways. Such control improves both reaction efficiency and selectivity. These advancements have enabled transition metal or photoredox catalysis to facilitate radical relay carbonylation of unactivated alkenes, resulting in transformations such as oxyalkylative carbonylation, aminoalkylative carbonylation, fluoroalkylative carbonylation, double carbonylation, and rearrangement carbonylation.SET-mediated carbonylation significantly enhances the sustainability and scalability of the carbonylation process by reducing reliance on precious metal catalysts and enabling milder reaction conditions. Additionally, by carefully controlling reaction intermediates, we have fine-tuned the process to produce a wide range of carbonylation products with high selectivity. This flexibility expands the applications of carbonylation in synthetic chemistry and industrial processes. Finally, we place particular emphasis on the application of carbonylation reactions in drug discovery, where they serve as powerful functional handles for the late-stage modification of bioactive molecules. The broad applicability of SET-mediated carbonylation methods to various chemical bonds significantly enriches the toolbox for drug synthesis, enabling the efficient functionalization of complex molecules. This versatile approach has the potential to accelerate the discovery of novel therapeutic agents, making it a critical tool in modern medicinal chemistry.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




References
-
- Wang Y.; Yang H.; Zheng Y.; Hu M.; Zhu J.; Bao Z.-P.; Zhao Y.; Wu X.-F. Carbon Monoxide Enabling Synergistic Carbonylation and (Hetero)aryl Migration. Nat. Catal. 2024, 7, 1065–1075. 10.1038/s41929-024-01204-6. - DOI
-
- Peng J.-B.; Wu F.-P.; Wu X.-F. First-Row Transition-Metal-Catalyzed Carbonylative Transformations of Carbon Electrophiles. Chem. Rev. 2019, 119, 2090–2127. 10.1021/acs.chemrev.8b00068. - DOI - PubMed
- Beller M.Catalytic Carbonylation Reactions; Springer: Berlin, 2006.
- Wu X.-F.; Neumann H.; Beller M. Synthesis of Heterocycles via Palladium-Catalyzed Carbonylations. Chem. Rev. 2013, 113, 1–35. 10.1021/cr300100s. - DOI - PubMed
- Friis S. D.; Lindhardt A. T.; Skrydstrup T. The Development and Application of Two-Chamber Reactors and Carbon Monoxide Precursors for Safe Carbonylation Reactions. Acc. Chem. Res. 2016, 49, 594–605. 10.1021/acs.accounts.5b00471. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

