Population Pharmacokinetic/Pharmacodynamic and Exposure-Response Modeling of Garadacimab in Healthy Volunteers and Patients With Hereditary Angioedema
- PMID: 40042097
- PMCID: PMC12072213
- DOI: 10.1002/psp4.70009
Population Pharmacokinetic/Pharmacodynamic and Exposure-Response Modeling of Garadacimab in Healthy Volunteers and Patients With Hereditary Angioedema
Abstract
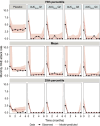
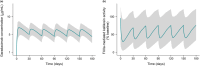
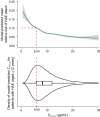
Hereditary angioedema (HAE) is a rare genetic disease that manifests as recurrent, unpredictable, and potentially life-threatening attacks of angioedema. Garadacimab is a first-in-class, fully human, monoclonal antibody targeting activated factor XII (FXIIa) that is under clinical development for the long-term prophylaxis of HAE attacks. We developed population pharmacokinetic (PK)/pharmacodynamic (PD)/exposure-response (ER) models using pooled data across clinical studies to quantify the relationship between garadacimab concentration and the relative risk of HAE attacks and to support the rationale for 200 mg once-monthly dosing. The PK of garadacimab was adequately characterized by a two-compartment model with first-order absorption and elimination. The PD, as analyzed by FXIIa-mediated kallikrein activity, was adequately characterized by a direct inhibitory response model. PK/PD parameters were generally consistent across multiple covariates. ER analysis based on a repeated-time-to-event model showed that administration of garadacimab 200 mg subcutaneously (SC) once monthly results in 75% of patients reaching the target therapeutic threshold (90% reduction in relative risk of attack vs. run-in). Use of a loading dose (two 200 mg SC injections) as the first administration achieved steady-state PK exposures and PD response, with 85% of patients having exposures surpassing the therapeutic threshold. The models support the use of garadacimab 200 mg SC once-monthly dosing in patients aged ≥ 12 years, with no need for dose adjustments, and indicate that, due to the achievement of garadacimab steady-state exposures after the first administration, the use of a loading dose may facilitate the early onset of protection against HAE attacks, as observed in clinical studies.
Keywords: exposure–response; garadacimab; hereditary angioedema; pharmacodynamics; pharmacokinetics.
© 2025 The Author(s). CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
R.G., M.W., C.J., and D.P. are full‐time employees of Metrum Research Group and were paid consultants for this work. S.C. and J.F. were full‐time employees of Metrum Research Group at the time the study was conducted and were paid consultants for this work. F.G. was a full‐time employee of CSL Behring at the time the study was conducted and a shareholder of CSL Limited. A.S., B.D.M.‐L., and P.N. are full‐time employees of CSL Behring LLC and shareholders of CSL Limited. J.‐P.L. and I.P. are full‐time employees of CSL Innovation GmbH and shareholders of CSL Limited. As an associate editor for
Figures


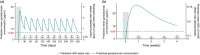
References
-
- Busse P. J., Christiansen S. C., Riedl M. A., et al., “US HAEA Medical Advisory Board 2020 Guidelines for the Management of Hereditary Angioedema,” Journal of Allergy and Clinical Immunology: In Practice 9 (2021): 132–150.e3. - PubMed
-
- Maurer M., Magerl M., Betschel S., et al., “The International WAO/EAACI Guideline for the Management of Hereditary Angioedema—The 2021 Revision and Update,” Allergy 77 (2022): 1961–1990. - PubMed
-
- Reshef A., Buttgereit T., Betschel S. D., et al., “Definition, Acronyms, Nomenclature, and Classification of Angioedema (DANCE): AAAAI, ACAAI, ACARE, and APAAACI DANCE Consensus,” Journal of Allergy and Clinical Immunology 154 (2024): 398–411. - PubMed
-
- Bygum A., “Hereditary Angio‐Oedema in Denmark: A Nationwide Survey,” British Journal of Dermatology 161 (2009): 1153–1158. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

