Effects of Therapeutic Antibiotic Exposure on the Oropharyngeal and Fecal Microbiota in Infants With Cystic Fibrosis
- PMID: 40042126
- PMCID: PMC11881217
- DOI: 10.1002/ppul.71024
Effects of Therapeutic Antibiotic Exposure on the Oropharyngeal and Fecal Microbiota in Infants With Cystic Fibrosis
Abstract
Background: Systemic antibiotics can impact all microbes inhabiting patients, regardless of the intended target organism(s). We studied the simultaneous effects on respiratory and fecal microbiomes of β-lactam antibiotics administered for respiratory symptoms in infants with cystic fibrosis (IWCF).
Objective: To compare the magnitude and duration of intended (respiratory) and unintended (fecal) antimicrobial action by analyzing oropharyngeal (OP) and fecal microbiota in IWCF.
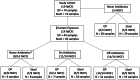
Design: Shotgun metagenomic sequencing and qPCR were performed on OP and fecal samples collected longitudinally from 14 IWCF (ages 1-17 months) during ("On Antibiotics") and after ("Off Antibiotics") β-lactam therapy, and from 5 IWCF (3-16 months) never treated with antibiotics.
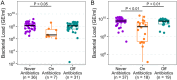
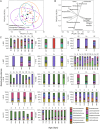
Results: Total bacterial loads (TBL) for On Antibiotics samples were lower than for both Never (OP and fecal) and Off Antibiotics samples (fecal only). α-diversities (within-sample) for OP On Antibiotics samples were lower than for Never and Off Antibiotics samples but did not differ between fecal sample groups. β-diversity (between-sample) differed between all OP sample groups and between fecal On and Never Antibiotics and Off and Never antibiotics samples; however, fecal On and Off Antibiotics sample β-diversities did not differ. Patterns of change in antibiotic resistance gene abundances reflected shifts in microbial community composition.
Conclusions: β-lactam antibiotic exposure was followed by marked alterations in both OP and fecal microbiota. While microbiota appeared to rebound after treatment in both sample types, our results suggest that fecal microbiota recovered less than OP. The clinical consequences of these findings should be studied in IWCF and other populations frequently treated with antibiotics.
Keywords: antibiotic resistance; metagenome; respiratory symptoms.
© 2025 The Author(s). Pediatric Pulmonology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Cystic Fibrosis Foundation Patient Registry , 2023 Annual Data Report (Cystic Fibrosis Foundation, 2024).
-
- Hoppe J. E., Hinds D. M., Colborg A., et al., “Oral Antibiotic Prescribing Patterns for Treatment of Pulmonary Exacerbations in Two Large Pediatric CF Centers,” Pediatric Pulmonology 55, no. 12 (2020): 3400–3406. - PubMed
-
- Sawicki G. S., Rasouliyan L., Pasta D. J., et al., Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis ., “The Impact of Incident Methicillin Resistant Staphylococcus aureus Detection on Pulmonary Function in Cystic Fibrosis,” Pediatric Pulmonology 43, no. 11 (2008): 1117–1123. - PubMed
-
- LeCleir L. K. and Pettit R. S., “Piperacillin‐Tazobactam Versus Cefepime Incidence of Acute Kidney Injury in Combination With Vancomycin and Tobramycin in Pediatric Cystic Fibrosis Patients,” Pediatric Pulmonology 52, no. 8 (2017): 1000–1005. - PubMed
-
- Carreno J., Smiraglia T., Hunter C., Tobin E., and Lomaestro B., “Comparative Incidence and Excess Risk of Acute Kidney Injury in Hospitalised Patients Receiving Vancomycin and Piperacillin/Tazobactam in Combination or as Monotherapy,” International Journal of Antimicrobial Agents 52, no. 5 (2018): 643–650. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

