Vasoconstriction-inhibiting factor: an endogenous inhibitor of vascular calcification as a calcimimetic of calcium-sensing receptor
- PMID: 40042167
- PMCID: PMC12038241
- DOI: 10.1093/cvr/cvaf016
Vasoconstriction-inhibiting factor: an endogenous inhibitor of vascular calcification as a calcimimetic of calcium-sensing receptor
Abstract
Aims: Patients with chronic kidney disease (CKD) show a high risk of cardiovascular diseases, predominantly caused by accelerated vascular calcification. Vascular calcification is a highly regulated process with no current treatment. The vasoconstriction-inhibiting factor (VIF) peptide was recently discovered with vasoregulatory properties, but no information regarding calcification has been described.
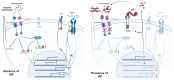
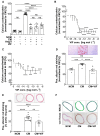
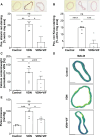
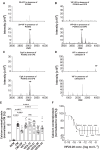
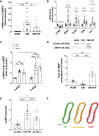
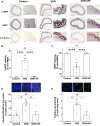
Methods and results: In the present work, the inhibitory calcification effect of the VIF peptide was analysed in vitro in vascular smooth muscle cells (VSMCs), ex vivo in rat aortic rings, as well as in vivo in rats treated with vitamin D and nicotine (VDN). The VIF peptide inhibits vascular calcification by acting as a calcimimetic for the calcium-sensing receptor, increasing carboxylated matrix Gla protein production and blocking the activation of calcification pathways. The VIF peptide decreased calcium influx, the production of reactive oxygen species, and the activation of multiple kinases in VSMCs. Furthermore, calcium deposition in the aortas of patients with CKD negatively correlates with the VIF peptide concentration. Moreover, we show the cleavage of the VIF peptide from chromogranin-A by 'proprotein convertase subtilisin/kexin type 2' and 'carboxypeptidase E' enzymes. In addition, 'cathepsin K' degrades the VIF peptide. The active site of the native 35 amino acid-sequence long VIF peptide was identified with seven amino acids, constituting a promising drug candidate with promise for clinical translation.
Conclusion: The elucidation of the underlying mechanism by which the VIF peptide inhibits vascular calcification, as well as the active sequence and the cleavage and degradation enzymes, forms the basis for developing preventive and therapeutic measures to counteract vascular calcification.
Keywords: CKD; CaSR; Vascular Calcification; inhibitor; smooth muscle cells.
© The Author(s) 2025. Published by Oxford University Press on behalf of the European Society of Cardiology.
Conflict of interest statement
Conflict of interest: A.A. has received payment or honoraria from Astra Zeneca. L.S. received research funding from Gnosis by Lesaffre, Bayer, Boehringer Ingelheim, not related to this work, and is a stockholder in Immuno diagnostic systems. T.S. has received a research grant from Else Kröner Fresenius. E.P.C.v.d.V., H.N. and J.J. are founding shareholders of AMICARE Development GmbH. R.V. is adviser to AstraZeneca, Glaxo Smith Kline, Fresenius Kabi, Novartis, Kibow, Baxter, Nipro, Fresenius Medical Care and Nextkidney. All other authors have no conflicting interests to declare.
Figures


References
-
- Alexopoulos N, Raggi P. Calcification in atherosclerosis. Nat Rev Cardiol 2009;6:681–688. - PubMed
-
- Chertow GM, Correa-Rotter R, Block GA, Drueke TB, Floege J, Goodman WG, Herzog CA, Kubo Y, London GM, Mahaffey KW, Mix TC, Moe SM, Wheeler DC, Parfrey PS. Baseline characteristics of subjects enrolled in the Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events (EVOLVE) trial. Nephrol Dial Transplant 2012;27:2872–2879. - PubMed
-
- Chen J, Budoff MJ, Reilly MP, Yang W, Rosas SE, Rahman M, Zhang X, Roy JA, Lustigova E, Nessel L, Ford V, Raj D, Porter AC, Soliman EZ, Wright J Jr, Wolf M, He J, Investigators C. Coronary artery calcification and risk of cardiovascular disease and death among patients with chronic kidney disease. JAMA Cardiol 2017;2:635–643. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

