The role of l-serine and l-threonine in the energy metabolism and nutritional stress response of Trypanosoma cruzi
- PMID: 40042273
- PMCID: PMC11934319
- DOI: 10.1128/msphere.00983-24
The role of l-serine and l-threonine in the energy metabolism and nutritional stress response of Trypanosoma cruzi
Abstract
l-Serine and l-threonine have versatile roles in metabolism. In addition to their use in protein synthesis, these amino acids participate in the biosynthesis pathways of other amino acids and even phospholipids. Furthermore, l-serine and l-threonine can be substrates for a serine/threonine dehydratase (Ser/ThrDH), resulting in pyruvate and 2-oxobutyrate, respectively, thus being amino acids with anaplerotic potential. Trypanosoma cruzi, the etiological agent of Chagas disease, uses amino acids in several biological processes: metacyclogenesis, infection, resistance to nutritional and oxidative stress, osmotic control, etc. This study investigated the import and metabolism of l-serine, l-threonine, and glycine in T. cruzi. Our results demonstrate that these amino acids are transported from the extracellular environment into T. cruzi cells through a saturable transport system that fits the Michaelis-Menten model. Our results show that l-serine and l-threonine can sustain epimastigote cell viability under nutritional stress conditions and stimulate oxygen consumption, maintaining intracellular ATP levels. Additionally, our findings indicate that serine plays a role in establishing the mitochondrial membrane potential in T. cruzi. Serine is also involved in energy metabolism via the serine-pyruvate pathway, which stimulates the production and subsequent excretion of acetate and alanine. Our results demonstrate the importance of l-serine and l-threonine in the energy metabolism of T. cruzi and provide new insights into the metabolic adaptations of this parasite during its life cycle.IMPORTANCETrypanosoma cruzi, the parasite responsible for Chagas disease, impacts 5-6 million individuals in the Americas and is rapidly spreading globally due to significant human migration. This parasitic organism undergoes a complex life cycle involving triatomine insects and mammalian hosts, thriving in diverse environments, such as various regions within the insect's digestive tract and mammalian cell cytoplasm. Crucially, its transmission hinges on its adaptive capabilities to varying environments. One of the most challenging environments is the insect's digestive tract, marked by nutrient scarcity between blood meals, redox imbalance, and osmotic stresses induced by the triatomine's metabolism. To endure these conditions, T. cruzi has developed a remarkably versatile metabolic network enabling it to metabolize sugars, lipids, and amino acids efficiently. However, the full extent of metabolites this parasite can thrive on remains incompletely understood. This study reveals that, beyond conventional carbon and energy sources (glucose, palmitic acids, proline, histidine, glutamine, and alanine), three additional metabolites (serine, threonine, and glycine) play vital roles in the parasite's survival during starvation. Remarkably, serine and threonine directly contribute to ATP production through a serine/threonine dehydratase enzyme not previously described in T. cruzi. The significance of this metabolic pathway for the parasite's survival sheds light on how metabolic networks aid in its endurance under extreme conditions and its ability to thrive in diverse metabolic settings.
Keywords: Trypanosoma cruzi; amino acid metabolism; bioenergetics; nutritional stress; transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

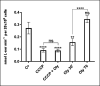
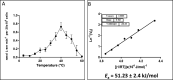








References
MeSH terms
Substances
Grants and funding
- 2021/12938-0/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 307487/2021-0/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)
- Centre National de la Recherche Scientifique (CNRS)
- ANR-19-CE15-0004/Agence Nationale de la Recherche (ANR)
- ANR-23-CE15-0040-01/Agence Nationale de la Recherche (ANR)
LinkOut - more resources
Full Text Sources
