Differential anaerobic oxidation of benzoate in Geotalea daltonii FRC-32
- PMID: 40042335
- PMCID: PMC11960108
- DOI: 10.1128/spectrum.02324-24
Differential anaerobic oxidation of benzoate in Geotalea daltonii FRC-32
Abstract
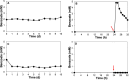
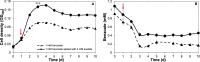
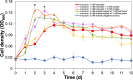
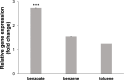
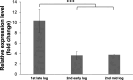
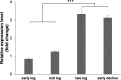
The efficient carbon source utilization in dynamic environments, including anoxic subsurface contaminated by aromatic compounds, is a challenge for anaerobic bacteria such as Geotalea daltonii strain FRC-32. The aim of this study was to elucidate the metabolic pathways employed by G. daltonii FRC-32 during anaerobic benzoate oxidation in the presence of acetate, a key intermediate in anaerobic organic matter degradation, to predict carbon source transport and utilization strategies. Simultaneous carbon source oxidation and monoauxic growth were observed in G. daltonii FRC-32 cultures grown on 1 mM benzoate + 5 mM acetate, 1 mM benzoate + 2 mM acetate, and 2 mM acetate spiked with 1 mM benzoate. Sequential carbon source oxidation and diauxic growth were observed only in cultures grown on 5 mM acetate spiked with 1 mM benzoate. Benzoate accumulation in G. daltonii FRC-32 whole cell lysates indicated that intracellular benzoate transport occurred during benzoate oxidation in the presence of acetate. Expression analyses of putative benzoate transporter BenK and protein-ligand binding affinity prediction suggested BenK's specificity for transporting benzoate. Relative expression levels for the gene benK, encoding BenK, and the genes bamNOPQ, involved in the benzoyl-CoA pathway, were significantly higher in cultures grown on both benzoate and acetate than in cultures grown on acetate as sole carbon source, indicating that intracellular benzoate accumulation facilitated the regulation of bamNOPQ. Our results demonstrated that G. daltonii FRC-32 can perform differential benzoate oxidation in the presence of acetate, by either simultaneous or sequential carbon source oxidation, which indicated the metabolic plasticity of G. daltonii FRC-32 in response to varying carbon source availability.IMPORTANCEThe contamination of anaerobic subsurface environments by crude oil derivatives including aromatic compounds is a global concern due to the persistence and toxicity of these pollutants. Anaerobic bacteria play a crucial role in the degradation of aromatic hydrocarbons under anoxic conditions; however, the potential mechanisms involved in metabolic regulation of aromatic degradation pathways are not well understood. This study contributed to elucidating how G. daltonii strain FRC-32 efficiently utilizes benzoate as a carbon source in the presence of acetate. Findings of intracellular benzoate accumulation and regulation of key genes associated with benzoate oxidation contributed to the understanding of G. daltonii FRC-32's aromatic degradation pathways, provided significant insights into potential mechanisms that modulate anaerobic benzoate oxidation in the presence of the energetically favorable carbon source acetate, and indicated metabolic strategies of G. daltonii FRC-32 in response to dynamic environmental conditions.
Keywords: Geotalea daltonii; acetate; benzoate; carbon source availability; differential anaerobic degradation; metabolic strategies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Bwapwa JK. 2022. Chapter 23 - Factors affecting the bioremediation of industrial and domestic wastewaters, p 461–472. In Das S, Dash HR (ed), Microbial biodegradation and bioremediation, Second Edition. Elsevier.
-
- Megharaj M, Venkateswarlu K, Naidu R. 2014. Bioremediation, p 485–489. In Wexler P (ed), Encyclopedia of toxicology, Third Edition. Academic Press, Oxford.
-
- Miles SM, Gestler R, Dworatzek SM. 2024. Bioremediation of petroleum hydrocarbons in the subsurface, p 479–502. In García-Rincón J, Gatsios E, Lenhard RJ, Atekwana EA, Naidu R (ed), Advances in the characterisation and remediation of sites contaminated with petroleum hydrocarbons. Springer International Publishing, Cham.
-
- Singh P, Singh VK, Singh R, Borthakur A, Madhav S, Ahamad A, Kumar A, Pal DB, Tiwary D, Mishra PK. 2020. Chapter 1 - Bioremediation: a sustainable approach for management of environmental contaminants, p 1–23. In Singh P, Kumar A, Borthakur A (ed), Abatement of environmental pollutants. Elsevier.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

