Burden and care time for dementia caregivers in the LIVE@Home.Path trial
- PMID: 40042468
- PMCID: PMC11881633
- DOI: 10.1002/alz.14622
Burden and care time for dementia caregivers in the LIVE@Home.Path trial
Abstract
Introduction: We investigated the effectiveness of the multicomponent learning, innovation, volunteer support, empowerment (LIVE) intervention on caregiver burden and care time in dyads of home-dwelling people with dementia and caregivers.
Method: A 24 month, multicenter, stepped-wedge trial, randomized dyads to receive the 6-month LIVE intervention by municipal coordinators (May 2019 to December 2021). Primary outcomes were caregiver burden assessed by Relative Stress Scale (RSS) and informal care time spent on personal activities assessed by Resource Utilization in Dementia Personal Activities of Daily Living (RUD-PADL). Analyses used an intention-to-treat.
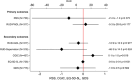
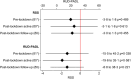
Results: Two hundred eighty dyads were enrolled. Caregivers during the intervention period reported lower levels of RSS of 0.7 points (standard deviation [SD]: 0.8) compared to the caregivers in the control period. Caregivers during the intervention period reported more time spent on PADL of 11.7 hours/month (SD: 8.7) compared to caregivers during the control period; both were not statistically significant (P > 0.05).
Discussion: The LIVE intervention did not reduce caregiver burden or care time.
Trial registration: ClinicalTrials.gov NCT04043364.
Highlights: Two hundred eighty persons with dementia and caregivers were included in a stepped wedge randomized controlled trial. We used the learning, innovation, volunteer support, empowerment (LIVE) intervention. The LIVE intervention did not reduce caregiver burden or informal care time. The LIVE intervention improved the caregiver's clinical global impression of change. Positive change was most pronounced for coordinator personalized support.
Keywords: care coordination; caregiver burden; dementia; resource use; stepped wedge randomized controlled trial.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dr. Ballard reports personal fees from Johnson and Johnson, Novo Nordisk, ReMynd, Acadia, AARP, Addex, Eli Lilly, GW Pharma, BMS, Janssen, Orion, Sunovion, Suven, Roche, Biogen, and TauRx. Dr. Selbæk reports honoraria for lectures at symposia sponsored by Eisai and Eli‐Lilly and has participated on advisory boards for Eli‐Lilly, Eisai, and Roche regarding disease‐modifying treatment of Alzheimer's disease. Dr. Aarsland reports grants from Roche Diagnostics, consulting fees from Roche Diagnostics, Eisai, Eli Lilly, and GSK. All other authors have no interests to disclose. Author disclosures are available in the supporting information.
Figures

References
-
- European Commision, Directorate‐General for Employment, Social Affairs and Inclusion . Long‐term care repport. Trends, challenges and opportunities in an ageing society. Volume II, Country profiles. Publications Office. 2021. Accessed July 2, 2024. doi:10.2767/677726 - DOI
-
- The World Health Organization and Alzheimer's Disease International . Dementia: a public health priority. 2012. Accessed July 2, 2024. ISBN 978 92 4 156445 8.
-
- Global Burden of Disease 2019 Dementia Forecasting Collaborators . Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the global burden of disease study 2019. Lancet Public Health. 2022;7(2):e105‐e25. doi:10.1016/S2468-2667(21)00249-8 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

