Parkinson-like wild-type superoxide dismutase 1 pathology induces nigral dopamine neuron degeneration in a novel murine model
- PMID: 40042537
- PMCID: PMC11882636
- DOI: 10.1007/s00401-025-02859-6
Parkinson-like wild-type superoxide dismutase 1 pathology induces nigral dopamine neuron degeneration in a novel murine model
Abstract
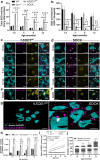
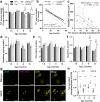
Atypical wild-type superoxide dismutase 1 (SOD1) protein misfolding and deposition occurs specifically within the degenerating substantia nigra pars compacta (SNc) in Parkinson disease. Mechanisms driving the formation of this pathology and relationship with SNc dopamine neuron health are yet to be fully understood. We applied proteomic mass spectrometry and synchrotron-based biometal quantification to post-mortem brain tissues from the SNc of Parkinson disease patients and age-matched controls to uncover key factors underlying the formation of wild-type SOD1 pathology in this disorder. We also engineered two of these factors - brain copper deficiency and upregulated SOD1 protein levels - into a novel mouse strain, termed the SOCK mouse, to verify their involvement in the development of Parkinson-like wild-type SOD1 pathology and their impact on dopamine neuron health. Soluble SOD1 protein in the degenerating Parkinson disease SNc exhibited altered post-translational modifications, which may underlie changes to the enzymatic activity and aggregation of the protein in this region. These include decreased copper binding, dysregulation of physiological glycosylation, and atypical oxidation and glycation of key SOD1 amino acid residues. We demonstrated that the biochemical profile introduced in SOCK mice promotes the same post-translational modifications and the development of Parkinson-like wild-type SOD1 pathology in the midbrain and cortex. This pathology accumulates progressively with age and is accompanied by nigrostriatal degeneration and dysfunction, which occur in the absence of α-synuclein deposition. These mice do not exhibit weight loss nor spinal cord motor neuron degeneration, distinguishing them from transgenic mutant SOD1 mouse models. This study provides the first in vivo evidence that mismetallation and altered post-translational modifications precipitates wild-type SOD1 misfolding, dysfunction, and deposition in the Parkinson disease brain, which may contribute to SNc dopamine neuron degeneration. Our data position this pathology as a novel drug target for this disorder, with a particular focus on therapies capable of correcting alterations to SOD1 post-translational modifications.
Keywords: Copper deficiency; Mouse model; Neurodegeneration; Oxidative stress; Parkinson disease; Post-translational modification; Protein misfolding; Substantia nigra pars compacta; Superoxide dismutase 1.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare no competing interests. Ethical approval and consent to participate: All experimental procedures involving the use of human post-mortem tissues were approved by the University of Sydney Human Research Ethics Committee (Approval NO. 2019/309). All experimental procedures involving the use of mice conformed to the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes, with protocols approved by the Animal Ethics Committee at the University of Melbourne (Ethics ID: 1814531.3) and ratified by the University of Sydney Animal Ethics Committees. Consent for publication: All authors read and approved the final manuscript prior to publication.
Figures





References
-
- Basso M, Massignan T, Samengo G, Cheroni C, De Biasi S, Salmona M et al (2006) Insoluble mutant SOD1 is partly oligoubiquitinated in amyotrophic lateral sclerosis mice. J Biol Chem 281:33325–33335. 10.1074/jbc.M603489200 - PubMed
-
- Basso M, Pozzi S, Tortarolo M, Fiordaliso F, Bisighini C, Pasetto L et al (2013) Mutant copper-zinc superoxide dismutase (SOD1) induces protein secretion pathway alterations and exosome release in astrocytes: implications for disease spreading and motor neuron pathology in amyotrophic lateral sclerosis. J Biol Chem 288:15699–15711. 10.1074/jbc.M112.425066 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

