Human brain tissue with MOGHE carrying somatic SLC35A2 variants reveal aberrant protein expression and protein loss in the white matter
- PMID: 40042641
- PMCID: PMC11882685
- DOI: 10.1007/s00401-025-02858-7
Human brain tissue with MOGHE carrying somatic SLC35A2 variants reveal aberrant protein expression and protein loss in the white matter
Abstract
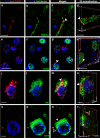
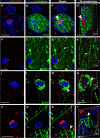
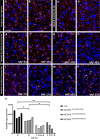
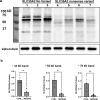
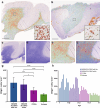
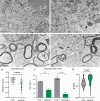
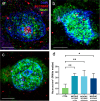
Mild Malformation of Cortical Development with Oligodendroglial Hyperplasia in Epilepsy (MOGHE) is a recently described disease entity primarily affecting young children with drug-resistant epilepsy, mainly affecting the frontal lobe. The condition is histopathologically defined by focal lesions with patchy areas of increased oligodendroglial cell density at the grey-white matter boundary and heterotopic neurons in the white matter. Approximately half of the individuals with MOGHE carry brain somatic variants in the SLC35A2 gene, which affects the UDP-galactose transporter and thus sphingolipid glycosylation. To investigate the impact of SLC35A2 variants on protein expression, we analysed MOGHE brain tissue with and without SLC35A2 mosaicism, distinguishing missense from nonsense variants. We developed an antibody targeting the N-terminus of the SLC35A2 galactose transporter and applied it for immunofluorescence (IF) analyses in a MOGHE cohort comprising 59 genetically tested individuals selected from three centres in Germany. The cohort included 13 individuals with SLC35A2 missense variants and 15 with SLC35A2 nonsense variants. Our findings confirm the localisation of the SLC35A2 protein in the Golgi apparatus of all neuroepithelial cell types as well as within Golgi outposts along oligodendroglial processes. The protein distribution was altered in MOGHE samples dependent on the SLC35A2 variant and its allelic frequency. Western blot and IF analyses revealed a significant SLC35A2 reduction in MOGHE tissues carrying nonsense variants. Ultrastructural analyses from three MOGHE samples demonstrated hypomyelination in regions with increased oligodendroglial cell densities, regardless of the harbouring of SLC35A2 variants. Notably, this hypomyelination pattern decreased with age. These results suggested a role for the SLC35A2 protein in the pathogenesis of MOGHE and indicated the presence of additional myelin-associated pathomechanisms in those individuals who do not carry a pathogenic SLC35A2 variant.
Keywords: Brain; Epilepsy; MOGHE; Myelin; Neuropathology; Oligodendrocytes.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: The authors declare that there are no competing interests.
Figures



References
-
- Aledo-Serrano A, Valls-Carbo A, Fenger CD, Groeppel G, Hartlieb T, Pascual I et al (2023) d-galactose supplementation for the treatment of mild malformation of cortical development with oligodendroglial hyperplasia in epilepsy (MOGHE): a pilot trial of precision medicine after epilepsy surgery. Neurotherapeutics 20:1294–1304. 10.1007/s13311-023-01395-z - PMC - PubMed
-
- Blumcke I, Aronica E, Miyata H, Sarnat HB, Thom M, Roessler K et al (2016) International recommendation for a comprehensive neuropathologic workup of epilepsy surgery brain tissue: a consensus Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 57:348–358. 10.1111/epi.13319 - PubMed
-
- Blumcke I, Budday S, Poduri A, Lal D, Kobow K, Baulac S (2021) Neocortical development and epilepsy: insights from focal cortical dysplasia and brain tumours. Lancet Neurol 20:943–955. 10.1016/S1474-4422(21)00265-9 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

