Real-world treatment patterns and clinical outcomes in patients with locally advanced or metastatic urothelial carcinoma in Germany: retrospective CONVINCE study
- PMID: 40042678
- PMCID: PMC11882626
- DOI: 10.1007/s00432-025-06131-y
Real-world treatment patterns and clinical outcomes in patients with locally advanced or metastatic urothelial carcinoma in Germany: retrospective CONVINCE study
Abstract
Purpose: CONVINCE is a retrospective medical chart review study that examined demographics, treatment patterns, and outcomes in patients who received first-line (1L) treatment for locally advanced or metastatic urothelial carcinoma (la/mUC) in Germany.
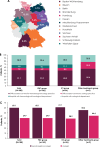
Methods: Eligible patients were adults with confirmed la/mUC who received any systemic 1L anticancer treatment between January 1, 2019, and September 30, 2021, outside of a clinical trial. Patients were grouped by type of 1L treatment: platinum-based chemotherapy (PBC), immune checkpoint inhibitor (ICI), or other treatments. Follow-up was ≥ 6 months after end of PBC or start of ICI or other treatments. The primary objective was measurement of real-world progression-free survival (rwPFS).
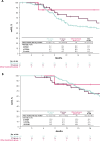
Results: Data were collected from 188 patients treated at 27 sites (hospitals or office-based practices). First-line treatment was PBC in 76.1% of patients, ICI in 19.1%, and other treatments in 4.8%. The most common PBC regimen was cisplatin + gemcitabine (72.7%), and the most common ICI was atezolizumab (44.4%); 4.2% of PBC-treated patients received avelumab 1L maintenance. In patients who received 1L PBC, ICI treatment, or other treatments, median (95% CI) rwPFS was 10.5 months (9.2-11.6), 12.6 months (8.9-22.9), and not evaluable; median (95% CI) real-world overall survival was 18.1 months (16.5-19.0), 15.9 months (11.1-24.5), and not evaluable; and objective response rates were 56.6%, 60.0%, and 83.3%, including complete response in 14.0%, 20.0%, and 0%, respectively.
Conclusion: PBC was the most common 1L treatment in patients with la/mUC in Germany, consistent with treatment guidelines. Future studies are needed to assess outcomes with newer treatments.
Keywords: Immune checkpoint inhibitors; Treatment outcome; Treatment patterns; Urothelial carcinoma.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: K.S. has served in speakers bureaus and consulting or advisory roles for AAA, Amgen, Apogepha, Astellas, AstraZeneca, Bayer, Bristol Myers Squibb (personal and institutional), Eisai, EUSA-Pharma, Fosanis, Ipsen, Janssen Cilag, Merck, MSD, Novartis (personal and institutional), Pfizer (personal and institutional), and Roche, and has received travel, accommodations, and expenses from Astellas, AstraZeneca, Bayer, Janssen Cilag, Ipsen, Merck, and Sanofi-Aventis. S.M. has served in consulting or advisory roles for Amgen, AstraZeneca, Bayer, BD, Bebig, Janssen-Cilag, MSD, Pfizer, and Sanofi-Aventis, and has participated in speakers bureaus for Amgen, Apogepha, Astellas, AstraZeneca, Bayer, BD, Bebig, Ipsen, Janssen-Cilag, Merck, MSD, Novartis, Pfizer, Sanofi-Aventis, and Takeda. T.K. has served in speakers bureaus and consulting or advisory roles for AstraZeneca, BMS, Boehringer-Ingelheim, GSK, Janssen, MSD, Roche, and Takeda. M.R., C.S., and A.E. have nothing to disclose. U.O. reports employment with Merck Healthcare Germany GmbH, Weiterstadt, Germany, an affiliate of Merck KGaA. S.G. reports employment with Merck Healthcare KGaA, Darmstadt, Germany when the study was conducted. M.K. reports employment with Merck Healthcare KGaA, Darmstadt, Germany and reports stock and other ownership interests with Merck, Novartis, and UCB. R.L. has served in consulting or advisory roles for AstraZeneca, Gilead, Janssen-Cilag, Merck, Omnicare, Sanofi, and Takeda; has served in a speakers bureau for Merck; and has provided project support for AbbVie, AstraZeneca, Bristol Myers Squibb, Gilead, Janssen-Cilag, Merck, and Pfizer. S.S. has nothing to disclose. Ethical approval: The study protocol was submitted to the independent ethical committee of the Chamber of Physicians in Northern Rhine, which confirmed that because data were fully anonymized, patient informed consent was not required. The protocol was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines.
Figures
References
-
- Astellas (2023) Padcev (enfortumab vedotin-ejfv). Prescribing information (US).
-
- Astellas (2024a) Padcev (enfortumab vedotin). Summary of product characteristics (European Medicines Agency).
-
- Astellas (2024b) European Commission approves Astellas’ PADCEVTM (enfortumab vedotin) in combination with KEYTRUDA® (pembrolizumab) for first-line treatment of advanced urothelial cancer. https://www.astellas.com/en/news/29371. Accessed 3 September 2024.
-
- Bristol Myers Squibb (2024a) Opdivo (nivolumab). Prescribing information (US).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

