Toward Realization of Bioorthogonal Chemistry in the Clinic
- PMID: 40042792
- PMCID: PMC11882664
- DOI: 10.1007/s41061-025-00495-y
Toward Realization of Bioorthogonal Chemistry in the Clinic
Abstract
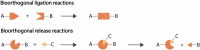
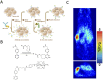
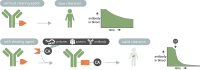
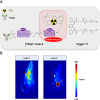
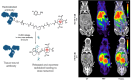
In the last decade, the use of bioorthogonal chemistry toward medical applications has increased tremendously. Besides being useful for the production of pharmaceuticals, the efficient, nontoxic reactions open possibilities for the development of therapies that rely on in vivo chemistry between two bioorthogonal components. Here we discuss the latest developments in bioorthogonal chemistry, with a focus on their use in living organisms, the translation from model systems to humans, and the challenges encountered during preclinical development. We aim to provide the reader a broad presentation of the current state of the art and demonstrate the numerous possibilities that bioorthogonal reactions have for clinical use, now and in the near future.
Keywords: Bioconjugation; Bioorthogonal chemistry; Bioorthogonal reaction; Click chemistry; Click-to-release; In vivo chemistry.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: K.d.R., R.R., and M.S.R. are employees of Tagworks Pharmaceuticals.
Figures









References
-
- Kolb HC, Finn MG, Sharpless KB (2001) Click chemistry: diverse chemical function from a few good reactions. Angew Chem Int Ed 40(11):2004–2021 - PubMed
-
- Saxon E, Bertozzi CR (2000) Cell surface engineering by a modified Staudinger reaction. Science 287(5460):2007–2010 - PubMed
-
- Tornoe CW, Christensen C, Meldal M (2002) Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem 67(9):3057–3064 - PubMed
-
- Rostovtsev VV et al (2002) A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl 41(14):2596–2599 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
