BIN1 reduction ameliorates DNM2-related Charcot-Marie-Tooth neuropathy
- PMID: 40042903
- PMCID: PMC11912451
- DOI: 10.1073/pnas.2419244122
BIN1 reduction ameliorates DNM2-related Charcot-Marie-Tooth neuropathy
Abstract
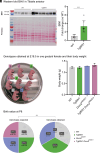
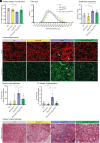
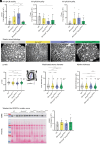
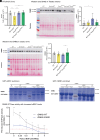
Charcot-Marie-Tooth (CMT) disease, the most common inherited neuromuscular disorder, manifests as progressive muscle weakness and peripheral nerve defects. Dominant mutations in DNM2, encoding the large GTPase dynamin 2, result in CMT without any suggested therapeutic strategy. Different dominant mutations in DNM2 also cause centronuclear myopathy (CNM), and increasing BIN1 (amphiphysin 2), an endogenous modulator of DNM2, rescued CNM in mice. Here, we found that increasing BIN1 level exacerbated the phenotypes of the Dnm2K562E/+ mouse carrying the most common DNM2-CMT mutation. Conversely, whole-body reduction of Bin1 expression level, through the generation of Dnm2K562E/+ mice with heterozygous loss of BIN1, restored motor performance and ameliorated muscle organization and structural defects of peripheral nerves. The rescue of motor defects was maintained at least up to 1 y of age. BIN1 inhibited the GTPase activity of DNM2, and the rescue was driven by an increased activity of the K562E DNM2-CMT mutant, and a normalization of integrin localization in muscle. Overall, this study highlights BIN1 as a modifier of DNM2-CMT, and its reduction as a potential therapeutic strategy. It also revealed an opposite pathological mechanism and inverse therapeutic concepts for DNM2-CMT peripheral neuropathy versus DNM2-CNM myopathy.
Keywords: Charcot–Marie–Tooth neuropathy; amphiphysin; dynamin; gene modulation; hereditary motor and sensory neuropathy.
Conflict of interest statement
Competing interests statement:Jocelyn Laporte is inventor on a patent describing BIN1 overexpression to cure centronuclear myopathies.
Figures

References
-
- Estévez-Arias B., et al. , Genetic approaches and pathogenic pathways in the clinical management of Charcot-Marie-Tooth disease. J. Transl. Genet. Genom. 6, 333–352 (2022).
-
- Laurá M., Pipis M., Rossor A. M., Reilly M. M., Charcot–Marie–Tooth disease and related disorders: An evolving landscape. Curr. Opin. Neurol. 32, 641–650 (2019). - PubMed
-
- Bolino A., D’Antonio M., Recent advances in the treatment of Charcot-Marie-Tooth neuropathies. J. Peripher. Nerv. Syst. 28, 134–149 (2023). - PubMed
-
- Züchner S., et al. , Mutations in the pleckstrin homology domain of dynamin 2 cause dominant intermediate Charcot-Marie-Tooth disease. Nat. Genet. 37, 289–294 (2005). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

