Direct Observation of Phase Change Accommodating Hydrogen Uptake in Bimetallic Nanoparticles
- PMID: 40042917
- PMCID: PMC11924317
- DOI: 10.1021/acsnano.4c18013
Direct Observation of Phase Change Accommodating Hydrogen Uptake in Bimetallic Nanoparticles
Abstract
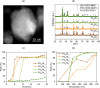
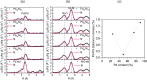
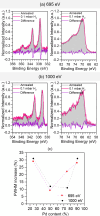
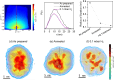
Hydrogen holds great promise as a cleaner alternative to fossil fuels, but its efficient and affordable storage remains a significant challenge. Bimetallic systems, such as Pd and Ni, present a promising option for storing hydrogen. In this study, using the combination of different cutting-edge X-ray and electron techniques, we observed the transformations of Pd-Ni nanoparticles, which initially consist of a NiO-rich shell surrounding a Pd-rich core but undergo a major transformation when they interact with hydrogen. During hydrogen exposure, the Pd core breaks into smaller pockets, dramatically increasing its surface area and enhancing the hydrogen storage capacity, especially in nanoparticles with lower Pd content. The findings provide a deep understanding of the morphological changes at the atomic level during hydrogen storage and contribute to designing cost-effective hydrogen storage using multimetallic systems.
Keywords: bimetallic nanoparticles; core−shell structure; hydrogen storage; in situ measurements; morphology changes.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Karpa W.; Grginović A. (Not so) Stranded: The Case of Coal in Poland. Energies 2021, 14, 8476.10.3390/en14248476. - DOI
-
- Mazloomi K.; Gomes C. Hydrogen as an Energy Carrier: Prospects and Challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. 10.1016/j.rser.2012.02.028. - DOI
-
- DOE Technical Targets for Onboard Hydrogen Storage for Light-Duty Vehicles. https://www.energy.gov/eere/fuelcells/doe-technical-targets-onboard-hydr... (accessed February 21st, 2025), 2017.
-
- Usman M. R. Hydrogen Storage Methods: Review and Current Status. Renew. Sustain. Energy Rev. 2022, 167, 11274310.1016/j.rser.2022.112743. - DOI
-
- Jena P. Materials for Hydrogen Storage: Past, Present, and Future. J. Phys. Chem. Lett. 2011, 2, 206–211. 10.1021/jz1015372. - DOI

