High-Salt Diet Increases Suprachiasmatic Neuronal Excitability Through Endothelin Receptor Type B Signaling
- PMID: 40042980
- PMCID: PMC11940741
- DOI: 10.1093/function/zqaf014
High-Salt Diet Increases Suprachiasmatic Neuronal Excitability Through Endothelin Receptor Type B Signaling
Abstract
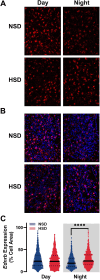
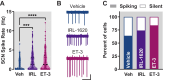
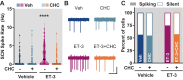
Circadian rhythms are 24-h oscillations in behavioral and biological processes such as blood pressure and sodium excretion. Endothelin B (ETB) receptor has been connected to the molecular clock in peripheral tissues and plays a key role in the regulation of sodium excretion, especially in response to a high-salt diet. However, little is known about the role of ETB in the primary circadian pacemaker in the brain, the suprachiasmatic nucleus (SCN), despite recent reports showing its enrichment in SCN astrocytes. In this study, we tested the hypothesis that high-salt diet (4.0% NaCl) impacts the circadian system via the ETB receptor at the behavioral, molecular, and physiological levels in C57BL/6 mice. Two weeks of high-salt diet feeding changed the organization of nighttime wheel-running activity, as well as increased the SCN expression of ETB mRNA determined by fluorescence in situ hybridization at night. Neuronal excitability determined using loose-patch electrophysiology was also elevated at night. This high-salt diet-induced increase in SCN activity was ameliorated by ex vivo bath application of an ETB antagonist and could be mimicked with acute treatment of endothelin-3. Finally, we found that the excitatory effects of endothelin-3 were blocked with co-application of an N-methyl-D-aspartate (NMDA) receptor antagonist, suggesting that glutamate mediates endothelin-induced neuronal excitability in the SCN. Together, our data demonstrate the presence of functional ETB receptors in SCN astrocytes and point to a novel role for endothelin signaling in mediating neuronal responses to a dietary sodium intake.
Keywords: astrocytes; circadian behavior; electrophysiology; endothelin; neurophysiology; sodium.
© The Author(s) 2025. Published by Oxford University Press on behalf of American Physiological Society.
Conflict of interest statement
D.M.P. holds the position of Editor-in-Chief for
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
