Differential regulation of BAX and BAK apoptotic activity revealed by small molecules
- PMID: 40043112
- PMCID: PMC11881913
- DOI: 10.1126/sciadv.adr8146
Differential regulation of BAX and BAK apoptotic activity revealed by small molecules
Abstract
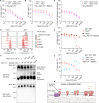
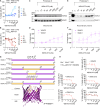
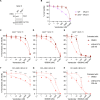
Defective apoptosis mediated by B cell lymphoma 2 antagonist/killer (BAK) or B cell lymphoma 2-associated X protein (BAX) underlies various pathologies including autoimmune and degenerative conditions. On mitochondria, voltage-dependent anion channel 2 (VDAC2) interacts with BAK and BAX through a common interface to inhibit BAK or to facilitate BAX apoptotic activity. We identified a small molecule (WEHI-3773) that inhibits interaction between VDAC2 and BAK or BAX revealing contrasting effects on their apoptotic activity. WEHI-3773 inhibits apoptosis mediated by BAX by blocking VDAC2-mediated BAX recruitment to mitochondria. Conversely, WEHI-3773 promotes BAK-mediated apoptosis by limiting inhibitory sequestration by VDAC2. In cells expressing both pro-apoptotic proteins, apoptosis promotion by WEHI-3773 dominates, because activated BAK activates BAX through a feed-forward mechanism. Loss of BAX drives resistance to the BCL-2 inhibitor venetoclax in some leukemias. WEHI-3773 overcomes this resistance by promoting BAK-mediated killing. This work highlights the coordination of BAX and BAK apoptotic activity through interaction with VDAC2 that may be targeted therapeutically.
Figures



References
-
- Lei I., Huang W., Noly P. E., Naik S., Ghali M., Liu L., Pagani F. D., Abou El Ela A., Pober J. S., Pitt B., Platt J. L., Cascalho M., Wang Z., Chen Y. E., Mortensen R. M., Tang P. C., Metabolic reprogramming by immune-responsive gene 1 up-regulation improves donor heart preservation and function. Sci. Transl. Med. 15, eade3782 (2023). - PMC - PubMed
-
- Anderson M. A., Tam C., Lew T. E., Juneja S., Juneja M., Westerman D., Wall M., Lade S., Gorelik A., Huang D. C. S., Seymour J. F., Roberts A. W., Clinicopathological features and outcomes of progression of CLL on the BCL2 inhibitor venetoclax. Blood 129, 3362–3370 (2017). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

