Oxytocin induces embryonic diapause
- PMID: 40043121
- PMCID: PMC11881891
- DOI: 10.1126/sciadv.adt1763
Oxytocin induces embryonic diapause
Abstract
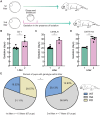
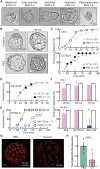
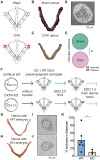
Embryonic development in many species, including case reports in humans, can be temporarily halted before implantation during a process called diapause. Facultative diapause occurs under conditions of maternal metabolic stress such as nursing. While molecular mechanisms of diapause have been studied, a natural inducing factor has yet to be identified. Here, we show that oxytocin induces embryonic diapause in mice. We show that gestational delays were triggered during nursing or optogenetic stimulation of oxytocin neurons simulating nursing patterns. Mouse blastocysts express oxytocin receptors, and oxytocin induced delayed implantation-like dispersion in cultured embryos. Last, oxytocin receptor-knockout embryos transferred into wild-type surrogates had low survival rates during diapause. Our results indicate that oxytocin coordinates timing of embryonic development with uterine progression through pregnancy, providing an evolutionarily conserved mechanism for ensuring successful reproduction.
Figures


References
-
- Renfree M. B., Fenelon J. C., The enigma of embryonic diapause. Development 144, 3199–3200 (2017). - PubMed
-
- Lopes L., Desmarais J. A., Murphy B. D., Embryonic diapause and its regulation. Reproduction 128, 669–678 (2004). - PubMed
-
- Renfree M. B., Shaw G., Diapause. Annu. Rev. Physiol. 62, 353–375 (2000). - PubMed
-
- Fenelon J. C., Banerjee A., Murphy B. D., Embryonic diapause: Development on hold. Int.J. Dev. Biol. 58, 163–174 (2014). - PubMed
-
- Norris M. L., Adam C. E., Concurrent lactation and reproductive performance in CFLP mice mated post partum. Lab. Anim. 15, 273–275 (1981). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

